Suresh Kumar Karri¹* and S. Srinivasan²
¹Department of Pharmacology, Annamalai University, Chidambaram India.
²Department of Pharmaceutics, Krupanidhi College of Pharmacy, Bangalore - 560 035 India.
Abstract
The present study was aimed to investigate the single dose effects of a herbomineral amino acid extract (HMAA) of Spinacia oleracea on alloxan induced diabetic nephropathy rats. In the present study, the animals were divided into 6 groups Group 1 – Normal saline as vehicle –control, Group 2 – Untreated diabetic rats (Alloxan induced), Group 3 – Animals treated with Metformin (0.5 mg/kg) – standard drug, Group 4 – Animals treated with Extract alone, Group 5 – Animals treated with Herbal + Mineral, Group 6 – Animals treated with Herbal + Mineral + Amino Acid. Based on the oxidative stress hypothesis of alloxan action, the role of herbal extract on free radicals in the pathology of diabetes mellitus has been studied in diabetic nephropathy rats. The present study demonstrated that herbal extract in the presence of an aminoacid (L-Arginine, 0.1 mM) and mineral (Zn+, in the form of Zinc Sulphate, 0.5 mM) could be a potent antioxidant and further, the data suggested that the mechanism underlying such protection is mediated via restoration of diabetic nephropathy leading to normalization of the diabetic rats.
Keywords
Spinacia oleracea; HMAA; anti-oxidant; diabetic nephropathy
Download this article as:| Copy the following to cite this article: Karri S. K, Srinivasan S. Effect of Herbomineral Amino acid Formulation on Wistar Strain Diabetic Nephropathy Rats. Biomed Pharmacol J 2011;4(1) |
| Copy the following to cite this URL: Karri S. K, Srinivasan S. Effect of Herbomineral Amino acid Formulation on Wistar Strain Diabetic Nephropathy Rats. Biomed Pharmacol J 2011;4(1). Available from: http://biomedpharmajournal.org/?p=1734 |
Introduction
Diabetes mellitus is a chronic metabolic disorder, characterized by disturbed glucose metabolism due to an absolute or relative insulin deficiency. As a consequence of the metabolic derangements in diabetes, various complications develop including both macro and micro vascular dysfunction1. New biochemical and molecular advances have contributed to a more profound understanding of the pathogenesis of diabetes and its complications2. Recently, increased oxidative stress has been proven to play a pivotal role in the etiology and pathogenesis of diabetes mellitus and its complications3. The role of oxidative stress in both type I and II diabetic mellitus is currently under intensive scientific investigation4-6. It is believed that insulin dependent diabetes mellitus (IDDM) results from the destruction of insulin producing pancreatic b cells by multiple factors including viruses, chemical toxins and autoimmune responses7-9.
It has been found that anti-oxidants, especially a-lipoic acid (a-LA) improved insulin sensitivity (Maddux et.al., 2001; Rudich et al., 1999; Jacon et. al., 1996). Spinacia oleracea has a-lipoic acid as one of its active ingredients (Dr. Duke’s Ethnobotanical databases). In view of the above studies and previous data showing that a-LA partially ameliorate diabetes related alternations in skeletal muscle glucose metabolism and PI-3 kinase /AKT activites (Bitar et al, 2004), it is aimed here to evaluate the hypoglycemic and antioxidant activities of aqueous and methanolic extract of a herbal extract (Spinacia oleracea). Plant drugs are frequently considered to be less toxic and free from side effects than synthetic ones. (Momin, 1987). In traditional methods, the medicinal plants being used, very often are in powder or paste forms of the crude herbs, which contain both the organic and inorganic constituents.
Zinc ion (Zn), an essential trace element with relatively low toxic profile in animals and humans. Life Science 75(2004), 741-751, (Underwood), has been proven to have a pronounced insulinomimetic activity. For example, Coulston and Dandona reported the insulinomimetic activity of Zn (II) ion 5 ´10-4 M in the isolated rat adipocytes in 1980 (Coulston and Dandona, 1980). Following this finding, the invivoantidiabetic effect of Zn (II) ion was reported by Shisheva et al. (1992), Chen et al (1998), and song et al (2001).
However experiments done so far on hypoglycemic herbs were mostly with their organic active principles. The role of some inorganic elements like potassium, zinc, calcium, manganese and traces of chromium in the improvement of impaired glucose tolerance and their indirect role in the management of DM are being increasingly recognized (Gusson and Saner, 1971; Mertz, 1981; Kinlse et. al., 1983; Niewochnn et al., 1986).
Besides, Zn ions are necessary for crystallization of insulin and insulin is stored as zinc complexes. Although many antidiabetic drugs from herb and mineral origin are used as a single medicine as well as in combination with other, these compound medicines secure to be more effective than a single drug.
Amino acids – Arginine and Leucine were found to have antidiabetic activity. (Pharmacology, Rang, Dale, H.M).In view of these, the present preliminary study was undertaken to investigate the single dose effect of herbal extract, a herbomineral extract and a herbominalaminoacid extract of methanolic form.
Materials and Methods
Collection of Plant Material
Whole plant Spinacia Oleracea plant powder were procured from the Nilgiri hills and were authentified by Botany Research Officer, Survey of Medicinal plants unit-siddha, Government Siddha Medical College Campus, Palayamkottai, Tamilnadu.
Preparation of leaf extracts by methanol using soxhlet apparatus
Methanolic extract was prepared by mixing 80gms of wet powder with 90% methanol. The residue was collected and dried on water bath at (60-80°C). The obtained extract was filtered concentrated and the residue is stored in refrigerator at 2-8°C for use in subsequent experiments. Male adult (Wistar strain) albino rats (150-200g). The animals were maintained on standard ratio and provided with clean drinking water ad libitum. The animals was kept in air-conditioned room (temperature 20 ±2C) and acclimatized for a period of 7 days. They were obtained from Animal House of RMMCH, Annamalai Nagar, Annamalai University.
The mineral zinc was taken in the form of salt zinc sulphate 0.5 mM and the amino acid was taken as L-arginin 0.1 mM in the present study.
Induction of Diabetes
A single dose of generally 125mg/kg (Intraperitonial) of alloxan monohydrate 5% dissolved in normal saline (warmed) was used for diabetes induction. Induction of Diabetes mellitus was confirmed after the 5th day of alloxan treatment of rats with blood glucose level ³ 150mg/dl was selected for the study.
Preparation of Diabetic Nephropathy Animals
The method of Yotsumoto et al., 1997 was used for experimental induction of diabetic nephropathy. After acclimatisation, overnight fasted animals were injected a bolus of alloxan (125 mg / kg of dissolved in 3 mM citrate buffer pH 4.5) intraperitoneally. Ten days after the single dose of alloxan injection, only those rats exhibiting plasma glucose levels
> 150 mg / dIat two occasions (with a gap of atleast 48 hours) were included in the study.
Acute oral toxicity study and LD50 determination
The procedure we followed was by Using OECD guidelines (organization of Economic Co-operation and Development) 423 (acute Toxic Class Method)
The method uses defined doses (100, 250, 500, 2000 mg/kg body weight) and the results allows a substance to be ranked and classified according to the Globally Harmonized systems (GHS) for the classification of chemical which cause acute toxicity. Twenty male wistar rats weighing 200-250 gms were used for study. The starting dose level of alcoholic extract of Spinacia oleracea was 2000mg/kg body weight p.o. Dose volume was administered 0.1ml/10 gm body weight to the rat which were fasted over night with water ad libitum. Food was withheld for a further 3-4 hours after administration of drug. Body weight of the rats before and after administration of the extract was noted and any changes in skin and fur, eyes and mucous membranes and also respiratory, circulatory, autonomic and central nervous system and somatomotor activity and behaviour pattern was observed, and also sign of tremors, convulsions, salivation, diarrhea, lethargy, sleep and coma were noted. The onset of toxicity and signs of toxicity were also noted, if any. LD50 was determined by Lithfied wilcoxsim method.
Gross Behavioural Studies
After an intrapertoneal administration one third of the same test dose calculated by (acute toxicity test) 210 ± .177 mg / kg b. wt of herbal extract to a group of 5 rats, each animal was observed for gross behavioural effects. The behaviour of the animals were continuously observed for 3 hours after administration of the herbal extract, then at the end of every 30 minutes for next 3 hours and finally after 24 hours.
Study Design
A total of 36 rats (Wistar Strain) were used for the present study and were divided into 6 groups with 6 animals in each group.
Group 1 – Normal saline as vehicle –control, Group 2 – Untreated diabetic rats (Alloxan induced), Group 3 – Animals treated with Metformin (.5 mg/kg) – standard drug, Group 4 – Animals treated with Extract alone, Group 5 – Animals treated with Herbal + Mineral, Group 6 – Animals treated with Herbal + Mineral + Amino Acid.
Superoxide Dismutase (SOD) and Catalase
The SOD activity was measured according to the method of Beauchamp and Fridovich. An adequate amount of the liver supernatant was mixed with the reaction mixtures, which contained 0.1 mM EDTA, 25 mM NBT, 0.1 mM xanthine, 50 mM sodium carbonate buffer (pH 10.2), and the final volume of the reaction mixture was brought up to 3 ml with distilled water. The reaction was initiated by the addition of 2 mU ml-1 xanthine oxidase and maintained under two 40 W lamps at 25°C. After 15 min, the inhibition rate of NBT reduction was spectrophotometrically determined at 560 nm. One unit of SOD is defined as the amount of enzyme required to reduce the NBT by 50%. The specific activity of SOD was expressed as a unit mg-1 protein in each supernatant and the values were calculated as a percentage of the control value.
This assay was conducted according to the method of Thomson et al. The liver supernatant was mixed with 2.8 ml of 50 mM phosphate buffer (pH 7.4). After equilibration at 30°C for 5 min, the reaction was started by the addition of 200 ml of 100 mM sodium perborate (pH 7.4). The catalase activity, which reduces the sodium perborate as a sustrate, was assessed spectrophotometrically following the consumption of hydrogen peroxide at 220 nm for 2 min. One unit of catalase was defined as the amount of enzyme required to reduce 1 mM of H2O2 min-1. The results were expressed as a unit mg-1 protein in each supernatant and the values were calculated as a percentage of the control value.
Glutathione
Cytosolic reduced glutathione (GSH) was measured using Glutathione Assay Kit from Cayman Chemicals according to the manufacturer’s instructions. The sample (100-150 mg) was deproteinized using phosphoric acid, and the amount of 5-thio-2-nitrobenzoic acid produced was measured in the supernatant.
Serum Creatinine and Pancreatic Nitrite Levels
The creatinine kit was procured from S.d fine chemicals and was manufactured by Sigma Diagnostic India Pvt. Ltd. Baroda.
The preparations were mixed well and allowed to stand at room temperature for exactly 20 minute and the absorbance was measured for blank, standard and test against distilled water on a autoanalyzer at 520 nm.
The serum creatinine values of 0.8-1.3 mg% were considered as normal.
Nitrite production was measured as 100 to 150-mg sample using Greiss reagent as described previously (Tanous, Veluthakal, Amin & Kowluri, 2002). The absorbance was measured at 540 nm, and the nitrite concentration was calculated from a sodium nitrite standard curve.
Urinary Albumin Levels
Albumin was measured by commercially available kits (based on quantitative colorimetric assay).
Dose Calculation
In all the animal studies the mineral zinc was taken in the form of salt, zinc sulphate 0.5 mM and the amino acid L-arginine was taken as 0.1mM and herbal extract to f70mg/kg b.wt (one third of LD50 value).
Zn SO4 – 0.5mM10
Molecular weight – 287.56, 287.56g – 1000ml – 1M, so for 0.5mM – 0.14378g
Arginine – 0.1mM – (Nakai M, Watanabe H, Satoh T et al., 1995)
Molecular weight – 210.67, 210.67g in 1000 ml –1M, so for 0.1mM – 0.021067g – 1000ml
Hence, every 2ml of herbal extract contain 17.5 mg of extract constitutents and 0.28 mg of ZnSO4 0.04 mg of L-Arginine.
Histopathology studies
A portion of the pancreas and kidney tissue, immediately after sacrifice, was fixed in 10% formalin. The washed tissue was dehydrated in the descending grades of isopropanol and finally cleared in Xylene. The tissue was then cut at 7 mm thickness, stained with haematoxylin and eosin. The sections were then viewed under tight microscope for histopathological change.
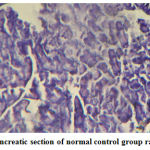 |
Figure 1: Pancreatic section of normal control group rat (´ 100) |
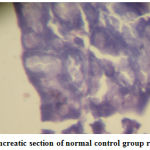 |
Figure 2: Pancreatic section of normal control group rat (´ 400) |
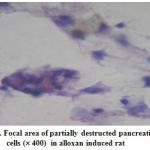 |
Figure 3: Focal area of partially destructed pancreatic |
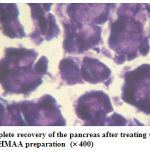 |
Figure 4: Complete recovery of the pancreas after treating the rat with the HMAA preparation (´ 400) |
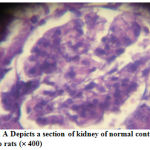 |
Figure 5: A Depicts a section of kidney of normal control group rats (´ 400) |
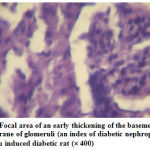 |
Figure 6: Focal area of an early thickening of the basement membrane of glomeruli (an index of diabetic nephropathy) in alloxan induced diabetic rat (´ 400) |
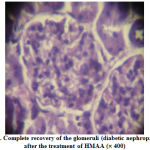 |
Figure 7: Complete recovery of the glomeruli (diabetic nephropathy) after the treatment of HMAA (´ 400) |
Table 1 : Gross Behavioural Effect
Observation
| S. No. |
Gross Activity | Upto 3 hrs | 3½ hrs | 4 hrs |
4½ hrs | 5 hrs |
6 hrs |
24 hrs |
| 1. | Respiration | ¯ | ¯ | ¯ | – | – | – | – |
| 2. | Writhing | + | + | – | – | – | – | – |
| 3. | Tremors | + | + | – | – | – | – | – |
| 4. | Convulsions | – | – | – | – | – | – | – |
| 5. | Salivation | – | – | – | – | – | – | – |
| 6. | Diarrohea | – | – | – | – | – | – | – |
| 7. | Mortality | – | – | – | – | – | – | – |
| 8. | Hind limb paralysis | – | – | – | – | – | – | – |
| 9. | Sedation | + | + | + | – | – | – | – |
| 10. | Skin irritation | – | – | – | – | – | – | – |
| 11. | Eye irritation | – | – | – | – | – | – | – |
| 12. | Any other effect | – | – | – | – | – | – | – |
¯¯ Strong depression ¯ Mild depression +Mild effect ++ Strong effect – Nil (no effect)
Table 2: Acute Toxicity Test
| Group | No. of Animal | Dose (mg / kg) |
Log dose | Dead / Total | % dead | % corrected | Probit |
| 1 | 5 | 100 | 2.000 | 0/5 | 0 | 8 | 3.36 |
| 2 | 5 | 125 | 2096 | 1/5 | 20 | 20 | 4.16 |
| 3 | 5 | 250 | 2.397 | 3/5 | 60 | 60 | 5.25 |
| 4 | 5 | 500 | 2.698 | 4/5 | 80 | 80 | 5.84 |
| 5 | 5 | 2000 | 3.301 | 5/5 | 100 | 95 | 6.64 |
Correction Formula: SE of LD 50 = 0.177 mg
Table 3: Effect of H+M+AA extract on SOD and Catalase
| Groups | SOD (UnitA / mg protein) |
Catalase (unitB / mg protein) |
||
| Liver | Kidney | Liver | Kidney | |
| Normal control | 6.80 ± 0.25 | 16.9 ± 1.38 | 75.2 ± 1.46 | 33.1 ± 1.64 |
| Diabetic control | 3.41 ± 0.09* | 10.1 ± 0.91* | 44.9 ± 1.38** | 21.1 ± 5.05** |
| Metformin | 6.48 ± 0.24* | 15.9 ± 1.37* | 65.9 ± 3.10** | 30.1 ± 2.43* |
| Herbal | 5.01 ± 0.34 | 14.6 ± 1.32 | 53.7 ± 2.94 | 26.8 ± 0.94 |
| H + M | 5.94 ± 0.20 | 13.7 ± 1.02 | 41.4 ± 0.95 | 23.1 ± 1.80 |
| H + M + AA | 5.81 ± 0.27 | 13.4 ± 0.47 | 40.3 ± 0.89 | 22.5 ± 1.75 |
* Value differ significantly at p < 0.05, ** Value differ significantly at p < 0.01
A – one unit of activity was taken as the enzyme reaction which gave 50% inhibition of nitrobluetetrazolium (NBT) reduction in one minute.
B – m moles of hydrogen peroxide consumed / min.
Table 4: Effect of H+M+AA on reduced GSH – in alloxan induced diabetic rats
GSH mg/100 g tissue
| Group | Liver | Kidney |
| Normal | 52.0 ± 4.38* | 33.7 ± 6.02* |
| Diabetic control | 28.0 ± 3.38** | 18.7 ± 1.70** |
| Std. (Metformin) | 46.3 ± 0.98* | 30.6 ± 1.96* |
| Herbal extract | 38.5 ± 4.35 | 24.0 ± 2.26 |
| H + Mineral | 32.6 ± 4.45* | 20.0 ± 2.84 |
| H + M + AA | 37.1 ± 31.4 | 28.3 ± 1.47 |
Values differ from each other significantly at * p < 0.05
** p < 0.01 Duncan’s Multiple Range Test was performed
Table 5: Serum Creatinine and Pancreatic Nitrite levels
| Groups | Serum Creatinine | Pancreatic Nitrite levels after (time) (in Umol / L) |
|||
| 10th day | 31st day | 24 hr | 48 hr | 72 hr | |
| Normal control | 41.5 ± 3.08 | 46.6 ± 3.05 | 240 ± 4.3 | 241 ± 3.8 | 240 ± 4.2 |
| Diabetic control | 46.8 ± 4.49 | 50.0 ± 1.67 | 377 ± 5.6 | 382 ± 6.9 | 771 ± 18.64 |
| Metformin | 42.8 ± 3.90 | 51.0 ± 5.36 | 280 ± 6.7 | 259 ± 7.1 | 243 ± 7.4 |
| Herbal (H) | 46.3 ± 4.63 | 42.3 ± 3.92 | 332 ± 4.9 | 344 ± 5.2 | 483 ± 11.46 |
| H + M | 45.3 ± 1.63 | 47.5 ± 3.08 | 309 ± 5.6 | 338 ± 4.7 | 407 ± 10.41 |
| H + M + AA | 46 ± 6.32 | 47.1 ± 7.54 | 298 ± 3.9 | 290 ± 4.4 | 276 ± 5.2 |
No significant difference observed between normal control and diabetic controls or the treated groups for serum creatinine throughout the duration of experiment.
Table 6: Urinary Albumin Excretion Levels Urine Albumin level from 0-40 day (mg/24hr)
| Groups | 0th day | 10th day | 20th day | 30th day | 40th day | Mean rise in UAE levels from 0 – 40 |
| Normal control | 170.83 ± 21.01 | 168.83 ± 20.02 | 172.14 ± 19.26 | 173.14 ± 23.46 | 172.16 ± 17.42 | 171.41 ± 16.23 |
| Diabetic control | 1055.33 ± 18.6 | 1552.66 ± 48.68 | 1504.8 ± 150.28 | 1488.6 ± 102.27 | 1411.33 ± 22.69 | 387 ± 70.9 |
| Std (Metformin) | 1079.16 ± 52.65 | 1449.83 ± 72.41 | 1410.5 ± 47.60 | 1406.33 ± 15.76 | 1277.5 ± 18.9 | 198.33 ± 63.74 |
| Herbal | 1054.66 ± 20.39 | 1527 ± 36.55 | 1510 ± 59.294 | 1420 ± 82.62 | 1323.66 ± 17.23 | 269.1 ± 10.64 |
| Herbal + Mineral | 1097.16 ± 0.87 | 1465.5 ± 59.19 | 1451.5 ± 74.54 | 1399 ± 54.13 | 1301 ± 90.54 | 204.3 ± 23.16 |
| Herbal + Mineral + Amino acid | 1043.5 ± 29.69 | 1481 ± 51.21 | 1501 ± 60.12 | 1437 ± 79.12 | 1214.5 ± 18.9 | 171.4 ± 18.21 |
* Herbal extract has better nephropathic protective action than standard metformin.
UAE – Urinary Albumin Excretion
H – Herbal extract
M – Mineral (Zinc sulphate – 0.5 mM)
AA – Amino acid (L – arginine – 0.1 mM)
Results
Gross behavioural effect
There was a mild respiratory depression after the administration of herbal extract for 4 hours. The writhing and tremors were also observed for 3 hrs of herbal administration. Methanolic extract had not produced any eye or skin irritation, but slight sedation was observed for the first 4 hours (Table-1).
Acute Toxicity Studies
In the result of acute toxicological observation the various test concentrations of the Spincia oleracea revealed that the percentage of death is directly proportional to the concentration of extract. The lower concentration (75mg / kg) did not show any effect and the other concentrations (125, 250, 500, 2000 mg/kg) showed a gradually increasing percentage of death. Hence from the results it is clearly inferred that the lower dose (125 mg) was the minimal lethal dose. The signs elicited by mice were hind limb paralysis, tremor, convulsion, laboured breathing started, respiratory failure and cardiac arrest. The cause of death was by respiratory failure and cardiac arrest. From the graph the LD50 dose of extract was found to be 210 mg/kg which was calculated graphically by interpolating against the probit value 5. Since the standard error (SE) was calculated as 0.177, the corrected LD50 value was 210 ± 0.177 mg/kg (Table-2).
SOD and Catalase
To avoid redox imbalance and oxidative damage, aerobic organisms possess an efficient biochemical defense system such as enzymatic (SOD, CAT and GSH), but these antioxidant enzymes do not completely protect from attack of ROS in conditions of severe oxidative stress. Values differ significantly at p < 0.05 (liver) and at p < 0.01 (kidney) (Table-3).
Glutathione
Duncan’s Multiple Range Test was performed. Values differ from each other significantly at p < 0.05 (Table-4).
Serum Creatinine and Pancreatic Nitrite level
No significant difference observed between normal control and diabetic controls or the treated groups for serum creatinine throughout the duration of experiment (Table-5).
Urinary Albumin Levels
Herbal extract has a significant (p < 0.05) protective action than standard metformin (Table-6).
Histopathology
The histopathological interpretations were made through light microsocpic observation. The targeted oxygen of kidney and pancrease were affected by the alloxan treatment at a dose of 125 mg/kg b.wt. The pancreatic sections revealed a partial destruction of the cells which confirms the induction of type-2 diabetic in the animal groups (fig.3).
The animal which received the HMAA preparation had restored back to the normal which was revealed from the fig. 4.
The normal kidney section could be observed in fig-5, the alloxan induced diabetic nephropathy condition was depicted in fig.6 as seen through the glomerular thickening. Fig-7 showed complete amelioration of diabetic nephropathy condition after the treatment with HMAA.
These histopathological studies once again confirm the induction of type –2 diabetes with alloxan at a dose of 125 mg/kg b.wt. Also, they proved that the HMAA extract treated groups have recovered from the diabetic nephropathic condition completely.
Discussion
The exact cellular mechanism of b-cell destruction remains unclear. However, it has been established that locally produced Reactive Oxygen Species (ROS) and Nitric Oxide (NO) induced after cytokine stimulation are involved11,12. Recent studies by Kanetoet al.13 and Matsuoko et al14 have proven that ROS lead to damage of b-cell through the induction of apoptosis and suppression of insulin biosynthesis.
Similarly, the development of type II diabetes has been associated with pancreatic b-cell dysfunction, and once hyperglycemia becomes apparent, b-cell function progressively deteriorates15.
Previous studies, have shown that sustained hyperglycemia, a characteristic of diabetes, increases intracellular ROS in pancreatic b-cell hence leading to cellular dysfunction16,17. Pancreatic b-cells are particularly susceptible to the deleterious effects of ROS because of their low expression of the antioxidant enzymes genes as compared to other tissues18,19. Hence, the cellular antioxidant status is an important determinant of its susceptibility to oxidative damage.
Glutathione (GSH) is an endogenous antioxidant that acts as a first line defense system against prooxidant status20. Anathanet al21 showed a significant reduction in plasma GSH levels in experimental diabetic rats. Similarly, depleted GSH levels have been repeatedly reported in several tissues of experimental diabetic animals, including eye, aorta, kidney as well as small intestine22, 24. Furthermore, significant decreases in plasma as well as erythrocyte GSH levels have been documented in diabetic patients25,26.
Zinc is an essential trace element necessary for normal protein metabolism, for the function of more than 200 give metalloenzyme, and for a host of physiologic functions. A poor zinc status is common in both liver cirrhosis and diabetes mellitus. Many of the clinical features of liver cirrhosis and diabetes mellitus have been linked to zinc deficiency. Kurt Grangreiff, Dirk Seinhold – have reported earlier that zinc supplementation improved in patients with liver cirrhosis and hepatic encephalopathy with and without diabetes mellitus neurological symptoms and signs of malnutrition. They also reported that zinc supplementation increased glucose disposal.
The data presented revealed a marked protective effect of herbal extract on alloxan-induced elevation of total nitrate/ nitrite level in pancreatic tissue. Whereby, concurrent treatment with H+M+AA normalized the pancreatic NO levels.
Diabetic animals treated with extract showed a significant reducing in the pancreatic nitrate / nitrite level. Such effect was obvious at both doses used following 48 h as well as 72 h.
In diabetes, hypoinoulinsulinemia increase the activity of the enzyme, fatty acyl coenzyme of oxides, which initiates b-oxidation of fatty acids, resulting in lipid peroxidation. Increased lipid peroxidation impairs membrane functions by decreasing membrane fluidity and changing the activity of membrane bound enzymes and reception.
Evidence is provided for the first time demonstrating that institution of herbal extract soon after induction of diabetes in rats can prevent proteinuria, kidney hypertrophy and increases in oxidative stress and nitrative stress. In support of the results presented here, microalbuminuria in diabetic patients is shown to disappear and the risk of developing nephropathy is reduced by herbal extract.
Its products are harmful (lipid radical and lipid peroxide) to the cell in the body and associated with atherosclerosis, brain and kidney damage (Soon and Tan, 2002).
Some studies elsewhere have indicated that plant extracts may contribute to body weight loss in animals (Bwitti et al., 2001). However, results of the current study suggest that oral administration of Spinacea oleracea leaf extract (along with mineral ZnSO4 and Amino acid L-arginine); did not significantly contribute to the changes in body weights since the loss in body weight of control alloxan-diabetic rats and treated diabetic rats did not significantly differ.
Body weight losses in diabetic rats as well as in type-1 diabetic patients have been previously reported (Bwilli et al., 2001) McDrmott, et al., 2003).
Recently it was also reported that diabetes mellitus, atherosclerosis and carcinogenesis could be caused by oxidation of membrane lipid mediated radicals such as superoxide or hydroxyl radical in humans. (Teacham R, Witztum J.L The oxidation hypothesis of atherosclerosis, Lancet 1994, 344: 793-5).
Therefore, many scientists have tried to obtain bioactive substances having the cytoprotective ability against cellular oxidative damage, as well as an enhancing ability of antioxidant enzymes activities (Jilal, Fuller et al 1993).
In these contexts, investigated whether HMAA can enhance the activities of SOD, CAT and GSH in alloxan induced diabetic rat.
The mean rise in Urinary Albumin Excretion (UAE) levels from day 0 to 40 in diabetic control was 387 ± 70.9 mg / 24 hr and it was significantly more in comparison to std, herbal, H + M, H + M + AA treated groups. Both herbal and metformin decreased the UAE levels significantly. However, the herbal extract treated group has shown a marked decreased in the mean UAE when compared to standard drug metformin.
In contrast, the H + M and H + M + AA treated groups brought down the UAE levels similar to that of standard metformin treated groups. Evidence is provided for the first time demonstrating that institution of HMAA soon after induction of diabetes in rats can prevent proteinuria, and increases in oxidative and nitrate stress. One of the possible neurochemicals that may have an important effect on this function is arginine varopressin. The cell bodies that secrete this neuro hormone are located in the paraveutricular nucleus of hypothalamus. (H.W. Schwartz, D.P. Figlewicz, O.G. Baskin, S.C. Woods, D.P. Porte, JR., Insulin and the cultural regulation of energy balance; update. Endocr. Rev. Monogr. 2 (1994) 109-113.
Conclusion
Based on the oxidative stress hypothesis of alloxan action, it was considered as an adequate model for investigating the role of free radicals in the pathology of diabetes mellitus. The present study demonstrates that herbal extract, a potent antioxidant, can exert anti-nephropathy effects by preserving pancreatic b-cell function. The data presented provide additional benefits of NO administration and may offer a promising natural and safe new trend for the prevention or delay of diabetic complications. In summary, the results of the present study indicate that herbal extract possess potent protective effect on the induction of diabetes by alloxan. The data provided suggest that the mechanism underlying such protection is mediated via prevention and restoration of antioxidant defense systems. These effects were completely reversed by standard drug metformin (except for the nephropathic condition), while treatment with Herbal + Mineral + AA produced partial to complete reversal. It is unclear at the present time if Herbal + M + AA treatment could provide for long-term benefits in treating diabetic nephropathy which would turn out as an end stage renal disease if left untreated. This will be the focus of future studies.
Acknowledgements
I express my heartful gratitude to Dr. R. Manavalan, M.Pharm., Ph.D., Professor and head, Department of Pharmacy, Annamalai University for providing necessary facilities for this research work. Also, I take this golden opportunity and immense pleasure to express my gratitude for the help rendered by Dr. K. Balamurugan, MD Pathology (Karaikal), for his valuable ideas and encouragement given to me in bringing this successful research work.
References
- Asulander W. Haire Joshu D, Houston C, Rhee C. Williams J. A controlled evaluation of staging dietary patterns to reduce the risk of diabetes in African-Amcrican women. Diabetes Care 2002;25:809-14.
- Dincer Y, Akcay T, Alademir Z. IIkova H. Effects of oxidative stress on glutathione pathway in red blood cells from patients with insulin independent diabetes mellitus. Metabolism 2002;51: 1360-2.
- Baynes J. Role of oxidative stress in the development of complications in diabetes. Diabetes 1991 ;40:405-12.
- Dandona P, Thusku K, Cook S, et al. Oxidative damage to DNA in diabetes mellitus. Lancet 1996;347:444-5.
- Mohamed A, Bierhaus A, Schiekofer S, Tritschler H. The role of oxidative stress and NF-kappa B activation in late diabetic complications. Biofactors 1999;10:157 -67.
- Wolf S. Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the etiology of diabetes mellitus and complications. Br Med Bull 1993;49:642-52.
- Alkinson M. Elsenbarth G. Type 1 I diabetes: new perspectives on disease pathogenesis and treatment. Lancet 2001 ;358:221-9.
- Bach J. Insulin-dependent diabetes mellitus as an autoimmune disease. Endocr Rev 1994; 15:516-.42.
- Jaeckel E. Manns M. Von Herrath M. Viruses and diabetes. Ann NY Acad Sci 2002;958:7-.25.
- Yali Zhary et al. Mechanism of oleic acid deterioration in insulin secretion: Role in the pathogenesis of type 2 diabetes. Life Science 77 (2005) 2071-2081.
- Rabinoviteh A. Suarez-Pinzon W, Strynadko K, Lakey J, Rajotle R. Human pancreatic islet beta cell destruction by cytokines involves oxygen free radicals and aldehyde production. J Clin Endocrinol Metab 1996;81 :3197-202.
- Sjoholm A. Aspects of the involvement of interleukin-I and nitric oxide in the pathogenesis of insulin-dependent diabetes mellitus. Cell Death Differ 1998;5:461-8.
- Kaneto H. Fujii J, Myint T, Miyazawa N, Islam K. Kawasaki Y, et al. Reducing sugars trigger oxidative modifications and apoptosis in pancreatic beta cells by provoking oxidative stress through the glycation reaction. Biochem J 1996;320:630-855.
- Matsuoka T, Kajimoto Y. Watada H. Kaneta H. Kashimoto M. Umayahara Y, et al. Glycation-dependent reactive oxygen species-mediated suppression of the insulin gene promoter activity in HITcells. J Clin Invest 1997;99: 144-50.
- Gorogawa S, Watada H. Kuroda A, Kawamori D. Yasuda T. Matsushia M. et al. Probucol preserves pancreatic beta cell function through reduction of oxidative stress in type 2 diabetes. Diabetes Res Clin Pract 2002;57:1.-10.
- Ihara Y, Toyokuni S. Uchida K. Odaka H, Tanaka T, Ikeda H. et al. Hyperglycemia causes oxidative stress in pancreatic beta cells of GK rats, a model of type 2 diabetes. Diabetes 1999;48:927-32.
- Nishikawa T, Edelstein 0, Du X. Yamagishi S. Matsumura T, Kaneda Y. et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycemic damage. Nature 2000;404:787-90.
- Lenzen S. Drinkgern J. Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med 1996;20:463-6.
- Zhang H, Ollinger K. Brunk U. Insulinoma cells in culture show pronounced sensitivity to alloxan-induced oxidative stress. Diabetologia 1995;38:635-41.
- Halliwell S, Gulteridge J. Free radicals in biology and medicine. 3rd Oxford: Clarendon Press; 1999.
- Anathan R, Basker C, NarmathaBai Y, Pani L, Ramkumas K.Antidiabetic effect of Gymnema montanum leaves: effect on lipid peroxidation induced oxidative stress in experimental diabetes. Pharmacol Res 2003;48:551-6.
- Bohr Y, Raghuram N, Sivakami S. Oxidative damage and altered antioxidant enzyme activities in the small intestine of streptozotocin-induced diabetic rats. Int J Biochem Cell Bioi 2004;36:89-97.
- Obrosova I, Fathallah L, Liu E, Nourooz-Zadeh J. Early oxidative stress in diabetic kidney: effect of DL-alpha lipoic acid. Free Radic Bioi Med 2003;34: 186-95.
- Yue K, Chung W, Leung A, Cheng C. Redox changes precede the occurrence of oxidative stress in eyes and aorta, but not in kidneys of diabetic rats. Life Sci 2003:73:2557- 70.
- Abou-Seif M, Youssef A. Evaluation of some biochemical changes in diabetic patients. Clin Chim Acta 2004;346: 161 70.
- Dincer Y, Akcay T, Alademir Z, IIkova H. Effects of oxidative stress on glutathione pathways in red blood cells from patients with dependent diabetes mellitus.’ Metabolism 2002;51: 1360-2.







