I. Onyesom¹* and I. C. Onyebuchi²
¹Department of Medical Biochemistry, Delta State University, Abraka Nigeria.
²Department of Biochemistry, Anambra State University, Uli Nigeria.
Abstract
The fact that a protein can by itself find its appropriate folded state in vitro does not necessarily mean that the same event occurs in vivo. There is evidence that all kinds of cells contain proteins known as chaperones which help in the proper assembly of protein structure. Molecular chaperones are essential proteins that bind to unfolded and partially folded polypeptide chains to prevent their improper association of exposed hydrophobic segments that might lead to non-native fold as well as polypeptide aggregation and precipitation. Their function is to “chaperone” polypeptide chains by apparently assisting them to fold; preventing proteins destined for membranes or for export through the membrane from folding instead of being inserted into the cell membrane; protect polypeptide chains from aggregation; induce misfolded proteins to fold to their normal conformations. There are three classes of molecular chaperones – the Hsp 70 family of 70kDa proteins which function as monomers; the chaperonins which are large multisubunit proteins; the Hsp 90 proteins which mainly participate in folding proteins involved in signal transduction such as steroid receptors. Co- chaperones are proteins that aid chaperones in carrying out their numerous functions. Interaction between these proteins (chaperones and co-chaperones) in the assembly of protein structure generally proceed via their binding to an unfolded or aggregated polypeptide’s exposed hydrophobic surfaces and subsequently releasing it, often repeatedly in a manner that facilitates its proper folding. Most molecular chaperones are ATPases and the favourable energy of ATP hydrolysis drives the chaperone’s bind-and-release cycle. Understanding of chaperone co-chaperone interactions in malarial infection is becoming useful in identifying potential targets for the development of novel anti-malarial drugs.
Keywords
Chaperone; malaria; polypeptide; cochaperones
Download this article as:| Copy the following to cite this article: Onyesom I, Onyebuchi I. S. Chaperone Co-Chaperone Interactions in Malarial Infection. Biomed Pharmacol J 2011;4(1) |
| Copy the following to cite this URL: Onyesom I, Onyebuchi I. S. Chaperone Co-Chaperone Interactions in Malarial Infection. Biomed Pharmacol J 2011;4(1). Available from: http://biomedpharmajournal.org/?p=1761 |
Introduction
Proteins fold to their native conformations in less than a few seconds. This is because proteins fold to their native conformation via directed pathways rather tumbling on them through random conformational searches. Thus as protein folds, its conformational stability increases sharply (i.e. free energy decreases sharply) which makes folding a one way process.
Under optimal experimental conditions, proteins often fold more slowly in vitro than they fold in vivo. This is because the folding protein form disulphide bond not present in the native proteins, which then slowly form native disulphide bonds through the process of disulphide interchange. Protein disulphide isomerase (PDI) catalyzes this process. RNase as an exception folds so much faster in vitro than in vivo as discovered by Anfinsens (Nelson and Cox, 2005).
Chaperones
Molecular chaperones are essential proteins that bind to unfolded and partially folded polypeptide chains to prevent the improper association of exposed hydrophobic segment that might lead to non-native fold as well as polypeptide aggregation and precipitation (Voet and Voet, 2006).
They are proteins that interact with partially folded or improperly folded polypeptides, facilitating correct folding pathways or providing microenvironment in which folding can occur (Nelson and Cox, 2005).
In molecular biology, chaperones are proteins that assist the non-covalent folding/unfolding and the assembly/disassembly of other macromolecular structures, but do not occur in these structures when the later are performing their biological functions. The functions of chaperones in protein folding are well illustrated in figure 1.1 indicating the partially folded intermediate at the junction between productive and off-pathway folding.
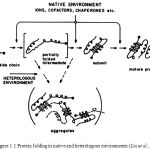 |
Figure 1.1: Protein folding in native and heterologous environments (Liu et al.,1996) |
Discovery of Molecular Chaperones
The classic experiments of Anfinsens demonstrated that all the information required for the folding of polypeptide chains into functional native three- dimensional conformations is encoded in their primary (Anfinsens, 1973). However, subtle changes in amino acid sequences, or in the milieu, can affect the folding process and the stability of the native state, often leading to the formation of stable inactive aggregate states (Gregersen et al., 2002). Protein aggregates are often toxic to cell (Taylor et al., 2002). Cells can express molecular chaperones and proteases to proteins, revert or eliminate aggregates (Sakahira et al., 2002).
The term “molecular chaperone” was initially used to describe how a nuclear protein, nucleoplasm, mediates the assembly of nucleosomes, but not take part in the final assembled complex (Laskey and Earnshaw, 1980). The term molecular chaperone was later extended to a group of highly conserved proteins, most of which stress induced are involved in the folding and assembly of other protein complexes in the cell (Ellis, 1987; Hemmingsen et al., 1988). Using mutagenesis, the Escherichia coli protein Dnak and GroEL were shown to be chaperones that control bacteria thermotolerance and participate in the proper folding and assembly of large protein complexes (Kusukawa and Yara, 1988). GroEL was shown assist the assembly of numerous self and recombinant proteins in E. coli cells (Goloubinoff et al., 1989; Van Dyk et al., 1989). These in vivo findings were confirmed in vitro with purified GroEL and GroES proteins, who significantly promoted in an ATP-dependent manner the correct refolding and assembly of urea-unfolded or acid-denatured RubisCO, into an active enzyme (Goloubinoff et al., 1989a).
As demonstrated by the essential role of GroEL in the cell at all temperatures (Fayet et al., 1989), molecular chaperones have important functions at physiological temperatures. In addition, the fact that most chaperone mutants are thermo-sensitive indicates that molecular chaperones assume vital functions under stress, such as repairing stress-induced damages in proteins (Mogk et al., 1999 and Paek and Walker, 1987). Overproduction of chaperones increases the tolerance of organisms to various stresses (Parsell and Lindquist, 1993) and, moreover, can suppress thermo-sensitive mutant phenotypes (Van Dyk et al., 1989). Deficiency in chaperone expression may reveal mutant phenotypes in populations, which otherwise exhibit wild type phenotypes. Molecular chaperones thus allow accumulation of conditional “silent” or “neutral” allelic mutations in populations, which become expressed only under the selective pressure of a stress (Queitsch et al., 2002) and Rutherford and Lindquist, 1998). This suggests that molecular chaperones can serve as buffer in the process of evolution (Rutherford and Lindquist, 1998).
Classification of Chaperones
There are many different families of chaperones, each family acts to aid protein folding in a different way. In a bacteria like E. coli, many of these proteins are highly expressed under conditions of high stress, for example, when placed in high temperatures. For this reason, the term “heat shock protein” has historically been used to name these chaperones. The prefix “Hsp” designates that the protein is a heat shock protein.
Classes of molecular chaperones in both prokaryotes and eukaryotes based on the way they aid protein folding include:
The Chaperonins, which are large multisubunit proteins.
Heat shock proteins;
The Hsp 70 family of 70kDa proteins which functions as monomers.
The Hsp 90 proteins, which are mainly involved with folding of protein involved in signal transduction such as steroid receptors.
Generally, they operate by binding to an unfolded or aggregated polypeptide’s solvent-exposed hydrophobic surfaces and subsequently releasing it often repeatedly in a manner that facilitates its proper folding.
ATP + H2O → ADP + Pi
Heat shock Protein
Heat shock Proteins (HSP) is a class of functionally related proteins whose expression is increased when cells are exposed to elevated temperatures or other stress. This increase in expression is transcriptionally regulated. The dramatic up regulation of the heat shock proteins is a key part of the heat shock response and is induced primarily by the heat shock factor (HSF). HSFs are found in virtually all living organisms, from bacteria to humans (Wu, 1995).
Heat shock proteins are named according to their molecular weight. For example, Hsp60, Hsp70 and Hsp90 (the most widely-studied HSPs) refer to families of heat shock protein on the order of 60, 70 and 90 kilodaltons in size, respectively (Schlesinger, 1990). The small 8 kilodaltons protein ubiquitin, which marks proteins for degradation, also has features of a heat shock (Raboy et al., 1991).
Hsp 70 system
Hsp 70 proteins generally have a molecular weight near 70,000 and are more abundant in cells stressed by elevated temperatures hence the name – ‘heat shock proteins’ (Nelson and Cox, 2005).
They are highly conserved 70KkDa monomeric proteins in prokaryotes and eukaryotes (Hsp 70 in eukaryotes and DnaK in prokaryotes), (Voet et al., 2006).
Although a precise mechanistic understanding has yet to be determined, it is known that Hsp70’s have a high affinity-bound state to unfolded proteins when bound to ADP, and a low affinity state when bound to unfolded to ADP. It is thought that many Hsp70s crowd around an unfolded substrate, stabilizing it and preventing aggregation until the unfolded molecules folds properly. At which time the Hsp70s lose affinity for the molecule and diffuse away. Hsp70 also acts as a mitochondrial and chloroplastic molecular chaperone in eukaryotes.
Functions of Hsp70 Protein
Hsp protein functions in association with the co-chaperone Hsp 40 to bind newly synthesized polypeptide at its hydrophobic regions as it emerges from the ribosome to prevent its premature folding.
They protect proteins that are denatured by heat by binding to the rich hydrophobic residues of the unfolded proteins thus preventing them from aggregation.
The Hsp proteins also block certain proteins that must remain unfolded until they have been translocated across the membrane and unfold proteins in preparation for their transport through the membrane.
They also help in the assembly of the oligomeric structure of protein in the association with Hsp 40.
Hsp 90 System
Hsp 90 (heat shock protein) is a molecular chaperone and is one of the most abundant proteins expressed in cells. It is a number of the heat shock protein family which is regulated in response to stress. Hsp90 is found in bacteria and all branches of eukarya, but is apparently absent in archea. Whereas cytoplasmic Hsp90 is essential for viability under all condition in eukaryotes, the bacterial homologue HtpG is dispensable under non-heat stress conditions.
Hsp90 (HtpG in E. coli) may be the least understood chaperone. Its molecular weight is about 90KD and it is necessary for viability in eukaryotes (possibly for prokaryotes as well). Heat shock protein 90 (Hsp90) is a molecular chaperone essential for activating many signaling proteins in the eukaryotic cell.
Each Hsp90 has an ATP-binding domain, a middle domain and a dimerization domain originally thought to clamp onto their substrate protein (also known as a client protein) upon binding ATP. The recently published structures by Vaughan at al., and Ali et al., indicate that client proteins may bind externally to both N-terminal and middle domains of Hsp90.
Hsp90 may also require co-chaperone like immunophilins, Sti L, p50 (Cdc37) and Ahai and also co-operates with the Hsp70 chaperone system.
The Hsp90 cycle (figure 1.2) represents a schematic diagram of protein assisted folding in Hsp90. X/Y represents an immature incompletely folded protein such as steroid receptor. Hsp40, Hsp70 and p23 are partner chaperones while Hop is a co-chaperone. Finally X-X represents a mature properly folded protein dinner.
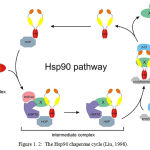 |
Figure 1.2: The Hsp90 chaperone cycle (Liu, 1998) Click here to View figure |
Chaperonins
Chaperonins are elaborate protein complexes required for the folding of a number of cellular proteins that do not fold spontaneously. In E. coli, an estimated 10-15% of cellular proteins require resident Chaperonin systems called GroEL/GroES. For under normal conditions, up to 30% require this assistance when the protein is heat stressed.
The protein first become known when they were found to be necessary for the growth of certain bacterial viruses (hence the designation “Gro”). Unfolded proteins are bound within pockets in the GroEL complex and the pockets are capped transiently by the GroES lid. GroEL undergoes substantial conformational changes, coupled to ATP hydrolysis and the binding and release of GroES, which promote folding of the bound polypeptide. Although the structure of the GroEL/GroES chaperonin is known, many details of its mechanism of action remain unsolved (Nelson and Cox, 2005)
Chaperonins possess pockets or grooves with which they bind to proteins. Figure 1.3 illustrates the surface and cut away images of the GroEL/GroES complexe. The cut-away (right) illustrates the large interior space within which the other proteins are bound.
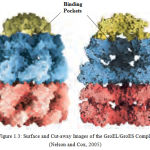 |
Figure 1.3: Surface and Cut-away Images of the GroEL/GroES Complex (Nelson and Cox, 2005)
|
Co-Chaperone
Co-chaperones are proteins that assist chaperones in protein folding and other functions. The activities of Hsp70 and Hsp 90 are modulated by co-chaperones. The Hsp70 co-chaperones Hip is a 50kDa cytosolic protein that was found to interact with the ATPase domain of Hsc70 and enhance Hsc70-substrate interactions by stabilizing its ADP-bound form (Hohfield et al., 1995).
Hop dis a unique co-chaperone that has the ability to interact with both Hsp70 and Hsp90 chaperones, thus providing a physical link between Hsp70 and Hsp90 chaperone machinery in various systems, such as the glucocorticoid and progesterone hormone receptors (Dittmar et al., 1996 and Chen and Smith, 1998).
Types of Co-Chaperone
There various types of co-chaperones of both bacterial and human importance. A list of a few co-chaperones includes:
GroES,Hch1, Aha1, Auxilin, BAG1
Hip (Hsc70 interacting protein)/ST13
Hop (Hsc70/Hsp organizing proteins)/STIP13
AHSA1
AHSA1, activator of heat shock 90kDa protein ATPase homolog 1(yeast), also known as AHSA1, is a human gene. AHSA1 has been shown to interact with heat shock protein 90kDa alpha (cytosolic), member A1.
BAG1
BCL2-associated anthanogene, also known as BAG1, is a human gene. The oncogene BCL2 is a membrane protein that blocks a step in a pathway leading to apoptosis or programmed cell death. The protein encoded by the gene binds to BCL2 and is referred to as BCL2-associated anthanogene. It enhances the anti-apoptotic effects of BCL2 and represents a link between the growth factor receptors and anti-apoptotic mechanisms (Liu et al., 1998).
BAG gene has been implicated in age related neurodegenerative diseases as Alzheimer’s disease. It has been demonstrated that BAG1 and BAG3 regulate the proteasomal and lysosomal protein elimination pathways respectively (Liu et al., 1998).
BAG3
BCL2-associating anthanogene 3 also known as BAG3, is a human gene. BAG proteins compete with the Hip for binding to the Hsc70/Hsp70ATPase domain and promote substrate release. All the BAG proteins have an approximately 45 amino acid BAG domain near the C terminus but differ markedly in their N-terminal regions. The protein encoded by this gene contains a WW domain in the N-terminal region and a BAG domain near the C terminus region. The BAG domain of BAG1, BAG2 and BAG3 interact specifically with the Hsc70 ATPase domain in vitro and in mammalian cells. All 3 proteins bind with high affinity to the ATPase domain of Hsc70 and inhibit its chaperone activity in a Hip-repressible manner. BAG gene has been implicated in age related neurodegenerative diseases as Alzheimer’s. It has been demonstrated that the BAG1 and BAG3 regulate the proteasomal and lysosomal protein elimination pathways (Takayama et al., 1999).
HOP
HOP (Hsp70/Hsp90 Organizing protein) belongs to the large group of co-chaperone, which regulate and assist the major chaperones (mainly heat shock proteins). It is one of the best studied co-chaperones of the Hsp70/Hsp90 complex. It was first discovered in yeast and homologues were identified in human, mouse, rat, insects, plants, parasites and virus. The family of these proteins is referred to as STI1 (stress inducible protein) and can be divided into yeast, plant and animal STI1 (Hop), (Johnson et al., 1998).
Hop (protein) has shown to interact with PRNP and heat shock protein 90kDa alpha (cytosolic), member A1 (Johnson et al., 1998).
Chaperone Co-Chaperone Interaction
As noted earlier, molecular chaperones are proteins that interact with folded or improperly folded polypeptides, facilitating correct folding pathways or providing microenvironment in which folding can occur.
Mechanism of Interaction
It is possible that chaperone protein recognize exposed helices or secondary structure element on their target proteins. This initial interaction may then allow the chaperone to guide or regulate the subsequent events in the folding as illustrated in figure 3.1 (Garrett and Grishams, 1999).
Interaction Types, Sites And Functions
Few amongst the numerous interactions between chaperones include:
Dnak and DnaJ interaction in coli (homolog of eukaryotic Hsp70 and Hsp40)
GroES and GroEL interaction in coli
Hsp90 and their Co-chaperones.
Dnak and DnaJ Interactions in E. coli
Interaction between the chaperone DnaK and its co-chaperone DnaJ have been implicated in proper protein folding in E. coli. The chaperones do not actively promote folding of the substrate protein, but instead prevent aggregation of unfolded peptides. For a population of polypeptides, some fractions of the polypeptides released at the end of the cycle are in the native conformation. The remainders are rebound by DnaK or are diverted to the chaperonin system. Figure 3.1 shows the interaction between the chaperone Dnak and its co-chaperone DnaJ
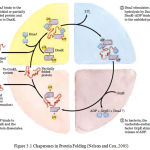 |
Figure 3.1: Chaperones in Protein Folding (Nelson and Cox, 2005)
|
The cyclic pathway by which chaperones bind and release polypeptides is illustrate d for the chaperone proteins DnaK and DnaJ.
GroES and GroEL Interactions in E. coli
In E. coli an estimated 10% to 15% of cellular proteins require the resident chaperone system called GroEL/GroES, for folding under normal conditions (up to 30% require this assistance when cells are heat stressed).
Unfolded proteins are bound within pockets in the GroEL complex and the pockets are capped transiently by the GroES “lid” (Figure 3.2). GroEl undergoes substantial conformational changes, coupled to ATP hydrolysis and the binding and release of GroES, which promote folding of the bound polypeptide. Although the structure of the GroEL/GroES chaperonin is known, many details of its mechanism of action remain unresolved (Nelson and Cox, 2005). This interaction is further illustrated in figure 3.2.
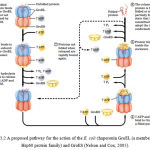 |
Figure 3.2: A proposed pathway for the action of the E. coli chaperonin GroEL (a member of the Hsp60 protein family) and GroES (Nelson and Cox, 2005)
|
Protein Misfolding and Treatment
The purpose of studying any human disease is to find ways to treat it. The story of protein folding has not yet led to treatments for the diseases involved, but this could happen within the next decade. The key is to find a small molecule, a drug that can either stabilize the normally found structure or disrupt the pathway that leads to a misfolded protein. Both thyroid hormone and the related compound TIP (2, 4, 6-triodophenol) can stabilize transthyretin. Since TIP neither blocks the action of thyroid hormone nor exerts any hormone-like effects of its own, it appears to be a promising treatment to FAP (King et al., 2001).
One of the few others currently publishing their research on small-molecule structure is working to stabilize p53, an acknowledged difficult target. In fact, one laboratory has obtained encouraging results by using two different approaches. Treatment based on our growing knowledge and continued researches of protein folding are on the way. When they arrive, the saga that begun with Pauling’s fundamental studies of protein structure and Anfinsen’s investigation of what some call the second genetic code, will reach its practical fruition (Kings et al., 2001)
Conclusion
The knowledge of molecular chaperones and co-chaperones interaction with respect to their associated function in protein folding have provided understanding into some disease pathogenesis. Chaperone co-chaperone interactions are being studied in P. falciparum malarial infection with the hope of identifying drug target.
References
- Pratt, C. W., Voet, D. and Voet, J. G. (2006). Protein: Three Dimensional Structure. Fundamental of Biochemistry; Life and Molecular Level. 2nd Ed. John Wiley and Sons LTD Honbken, USA Pp 161-170.
- Ellis, R. J. (2007). Proteins missassembly: Macromolecule Crowding and Molecular Chaperones. Adv. Exp. Biol. 594: 1-13
- Ellis, R. J. (2006). Molecular Chaperone: Assisting addition to folding. Trends in Biochemical Science. 31(7): 395-401
- Ellis, R. J. and Van der Vies S. M. (1991). Molecular Chaperone Manual. Review Biochemistry. 60:321-347
- King, F. W., Wawrzynow, A., Hohfiekd, J. and Zylics, M (2001). Co-chaperone BAG1 Hop and Hsp40 regulate 70 and Hsp90 interactions with wild type or mutant p53. The EMBO Journal. 20: 6297-6305.
- Garrett, R. H. and Grisham, C. M. (1999). Proteins: Secondary, Tertiary and Quaternary Structures. Biochemistry Life and Molecular Approach. 2nd Ed. Brooks/Cole-Thomas Pacific Groove, USA. Pp 171-205
- Johnson, B. D., Schumacher, R. J. Ross, E. D. and Toft, D. O. (1998). Hop Modulates Hsp70/Hsp90 Interactions in Protein Folding. J. Biol. Chem. 273 (6):3679-86.
- Liu, R., Takayama, S., Zheng, Y., Frosch, B., Chen, G. Q., Zhang, X., Reed, J. C. and Zhang, X. K. (1996). Interaction of BAG1 with Retinoic acid Receptor and its Inhibition of Retinoic acid-induced Apoptosis in Cancer Cells. J. Biol. Chem.273(27): 16985-92.
- Mathews, C. K. and Van Holde, K. E. (1996). Three Dimensional Structure of Protein. Biochemistry. 2nd Ed. University Press. Pp 193-195
- Nelson, D. L and Cox, M. M. (2005). Three Dimensional Structure of Protein. Lehninger Principles of Biochemistry. 4th Ed. W. H. Freeman and Company. New York, USA. Pp 149-153.
- Raboy, B., Sharon, G., Parag, H. A., Shochat, Y. and Kulka, R. G. (1991). Effect of Stress on Protein Degradation: role of the Ubiquitin system. Acta Biologica Hungarica42(1-3):3-20.
- Schlesinger. M. J. (1990). Heat Shock Proteins. The Journal of Biological Chemistry 265(21): 12111-12114
- Takayama, S., Xie, Z. and Reed, J. C. (1999). An Evolutionary conserved familt of Hsp70/Hsp70 Molecular Chaperone Regulators. J. Biol. Chem. 274(2): 781-6
- Tesic, M., Marsh, J. A., Cullinan, S, B. and Gaber, R. F. (2003). Functional Interactions between Hsp90 and the co-chaperones CNS1 and Cpr7 in Sacchromycees cerevisiae
- Won-chul S., Burkholder, W. F., Lu, C. Z., Zhao, X., Gottesman, M E. and Gross, C. A. (1998). Interaction of the Hsp molecular chaperone Dnak withits Co-chaperone DnaJ. http://www.pnas.org/content/95/26/15223.full Retrieved 31-12-09
- Wu, C. (1995). Heat Shock Transcription Factors: Structure and Regulation. Annual Review of Cell and Developmental Biology 11:441-69.







