Manuscript accepted on :August 25, 2010
Published online on: 24-11-2015
Plagiarism Check: Yes
Elias Babarbi, Maammar Haffas, Messaoud Guerri and Lakhdar Sekhri*
Department of Chemistry, Faculty of Sciences, University of Kasdi Merbah, Ouargla - 300 00 (Algeria).
Abstract
Tris (pentafluorophenyl) phosphine, bis (pentafuorophenyl) phenylphosphine, tris (4-fluorophenyl) phosphine and triphenylphosphine are readily reacted with an excess of methyl iodide in chloroform at boiling point to afford respectively phosphonium salts of methyl tris (pentafluorophenyl)phosphonium iodide, methyl bis (pentafuorophenyl)phenylphosphonium iodide, methyl tris (4-fluorophenyl) phosphonium iodide and methyl triphenylphosphonium iodide. These phosphonium salts showed significant activity against all the bacteria tested, Escherichia Coli, Protius, Staphylococcus Aureus and Pseudomonas. The combination of these phosphonium salts with the penicillin G or V gives a synergistic effect.
Keywords
(pentafluorophenyl); phosphine; synergistic effect; bacteria; penicillin-G; V
Download this article as:| Copy the following to cite this article: Babarbi E, Haffas M, Guerri M, Sekhri L. Synthesis of Some Fluorinated Phosphonium Iodide Derivatives; Study of their Biological Antibacterial Activities and the Effect of Synergy of these Compounds with Penicillin-G and Penicillin-V. Biomed Pharmacol J 2010;3(2) |
| Copy the following to cite this URL: Babarbi E, Haffas M, Guerri M, Sekhri L. Synthesis of Some Fluorinated Phosphonium Iodide Derivatives; Study of their Biological Antibacterial Activities and the Effect of Synergy of these Compounds with Penicillin-G and Penicillin-V. Biomed Pharmacol J 2010;3(2). Available from: http://biomedpharmajournal.org/?p=1506 |
Introduction
Since the 1940’s, chemists have developed all sorts of highly effective antibiotics (Sulfa drugs, Pinicillins, Tetracyclines, and others that are effective) against bacterial infections and viral infections. In recent years there has been a flood of papers describing the synthesis of new antibacterial compounds and isolation of some natural products and study of their biological antimicrobial activities penicillin derivatives,1 lignanan named crossandrin,2 triazolo-thiadiazinyl comarins,3 p-bromo and p-nitrophenylselenocyanate,4 pyrazoline-5-ones,5 thiacarbamides,6 substituted benzimidazole derivatives,7and others.8-10 In this introduction we have paid attention in penicillines and organophosphorus compounds.
The penicillin is one of the most widely used antibiotics and its antibacterial activity against both gram-positive and gram-negative organisms.11,12 The penicillin has two heterocyclic rings, the smaller, β-lactam, of which is crucial to biological activity and contain bulky side chains attached to the 6-aminopenicillanic acid.13-15
The establishment of the precise structure of penicillin was very difficult and was completed only in 1945, when Dorthy Hodgkin (Nobel Prize of chemistry 1964) brought the final tests thanks to work of diffraction of x-rays (Scheme-1) .
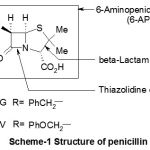 |
Scheme 1 |
The molecular skeleton lets foresee that it probably derives from two amino acids, in fact cysteine and valine what was indeed proven. Its general structure is shown in Scheme-2.
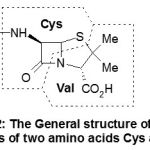 |
Scheme 2 |
There is also considerable interest in the synthesis and chemistry of organophosphorus compounds due to their utility in medicine and agricultural applications. Before World War II, there were almost no practical applications for synthetic organophosphorus compounds since they possessed exceedingly high levels of mammalian toxicity. Over the years, it was discovered that the mammalian toxicity levels could greatly reduced by proper structural modification, but that toxicity level to insects was very high. Since that time, Many organophosphorus compounds have been made and used in very large quantities in agriculture, not only as insecticides but also later as herbicides and in other applications. Medicinal organophosphorus chemistry is now an active and exciting area, with many opportunities for fresh research. High level anti cancer activity has been found in a number of phosphorus compounds such as cyclophosphamide-1, lysophosphatidyl choline-2, which are cytotoxic agaist tumor cells, and mitefosine-3, a choline phosphate (Scheme-3).
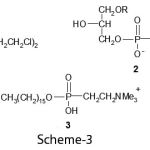 |
Scheme 3 |
In recent years, there have been numerous papers describing the synthesis and chemistry of phosphorus compounds, such as fluorovinyl-containing phosphines,16 and application due to their utility as corrosion inhibitors of iron,17,18
Now we found that the combination of penicillin G or V with phosphonium salts gives a synergistic effect and brought a high degree of antibacterial activity for this drug.
Results and Discussion
The strategy we have adopted for carrying this research consists of the following steps:
(i) synthesis of some fluorinated phosphonium iodide derivatives using Wittig reaction, this route has been widely used for the synthesis of alkenes and more recently, for the synthesis of phosphonium salts. Tris (pentafluorophenyl) phosphine 4a, bis (pentafuorophenyl) phenylphosphine 4a and tris (4-fluorophenyl) phosphine 4a are readily reacted with 1.3 equivalents of methyl iodide in chloroform at boiling point to afford respectively phosphonium salts of tris (pentafluorophenyl)phosphonium 5b iodide, bis (pentafuorophenyl)phenylphosphonium 5b and tris (4-fluorophenyl) phosphonium iodide 5b as shown in Scheme-4.
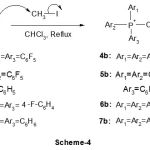 |
Scheme 4 |
(ii) study of their biological antibacterial activities and the effect of synergy of these compounds with penicillin G.
Experimental
Synthesis
General
19F, 31P{1H} and 1H NMR spectra were recorded on a Bruker DPX400 spectrometer operating at 376.5, 162.0 and 400.1 MHz, respectively. Peak positions are quoted relative to external CFCl3, 85%H3PO4 and TMS, respectively, using the high frequency positive convention. 13C{1H} NMR spectra (reference TMS) were recorded on a Bruker DPX 400 spectrometer, operating at 100.5 MHz. All NMR spectra were recorded using CHCl3 as internal standard. Fast atom bombardment (FAB) was recorded with a Crates MS50 with an m-nitrobenzyl alcohol matrix. Accurate mass determinations were carried out on a Kartos Concept IS spectrometer. Infra red spectra were recorded using a Perkin-Elmer 783 spectrometer equipped with a PE 600 data station. Melting points were determined using an Electrothermal melting point apparatus and were uncorrected. Silica thin layer chromatography (TLC) was conducted on percolated aluminium sheets (60 F254) with a 0.2 mm thickness (Aldrich Chemical Co.).
Synthesis of methyl tris (penta-fluorophenyl)phosphonium iodide 4a
A dry 250 ml, three-necked-round bottomed flask equipped with a rubber septum, a magnetic stirrer and reflux condenser was charged with tris (pentafluorophenyl) phosphine (2.5g, 3.76 mmol) in (50 mL) of chloroform. Methyl iodide (1.0 g, 0.5 mL, 7 mmol) was added over a 5 minutes period. The reaction mixture was refluxed on a water bath with gentle stirring for 3 hrs. The reaction is exothermic; care must be taken to ensure that the reaction is not too vigorous, by appropriate cooling. The mixture was transferred to a single rotavapor to leave colourless oil that crystallized on standing. This can, if required, recrystallised from propan-2-ol to give pure tris (pentafluorophenyl)phosphonium iodide (2.1 g, 3.12 mmol, 83%), m.p. 114-116°C. The material is sufficiently pure to suit most purposes. 1H NMR (400MHz; CDCl3): δ 1.05 (3H, d, CH3); 19F NMR (376.5MHz; CDCl3): δ -160 (6F, m, Ar-F ortho), -147(6F, m, Ar-F meta), -130 (3F, m, Ar-F para); 31P NMR (162 MHz; CDCl3): δ -74.35 (1P, dddd, J=34Hz, P); IR (KBr, cm-1): 2860-2980 (CH aliphatic), 1625 (C=C), 1250 (C-P), 1094 (C-F).
Synthesis of methyl bis (penta-fuorophenyl)phenylphosphonium 4b
A dry 250 ml, three-necked-round bottomed flask equipped with a rubber septum, a magnetic stirrer and reflux condenser was charged with bis (pentafuorophenyl) phenylphosphine (1.0 g, 2.26 mmol) in (50 mL) of chloroform. Methyl iodide (0.5 g, 0.25 mL, 3.5 mmol) was added over a 5 minutes period. The reaction mixture was refluxed on a water bath with gentle stirring for 2.5 hrs. The reaction is exothermic; care must be taken to ensure that the reaction is not too vigorous, by appropriate cooling. The mixture was transferred to a single rotavapor to leave colourless oil that crystallized on standing. This can, if required, recrystallised from propan-2-ol to give pure bis (pentafuorophenyl)phenylphosphonium iodide (1.12g, 1.92 mmol, 85%), m.p. 130-132°C. The material is sufficiently pure to suit most purposes. 1H NMR (400.1MHz; CDCl3): δ 1.35 (3H, d, J=28 Hz, CH3), 7.4 (3H, m, Ar-H, ortho and para), 7.9 (2H, t, Ar-H meta); 31P NMR (162MHz; CDCl3): δ -34.78 (1P, dd, J=45Hz, P); IR (KBr, cm-1): 2860-2980 (CH aliphatic), 1625 (C=C), 1225(C-P), 1150 (C-F).
Synthesis of methyl tris (4-fluoro-phenyl) phosphonium iodide 4c
A dry 250 ml, three-necked-round bottomed flask equipped with a rubber septum, a magnetic stirrer and reflux condenser was charged with tris (pentafluorophenyl) phosphine (2.0 g, 3.75 mmol) in (75 mL) of chloroform. Methyl iodide (1.0g, 0.5 ml, 7 mmol) was added over a 5 minutes period. The reaction mixture was refluxed on a water bath with gentle stirring for 3 hrs. The reaction is exothermic; care must be taken to ensure that the reaction is not too vigorous, by appropriate cooling. The mixture was transferred to a single rotavapor to leave colourless oil that crystallized on standing. This can, if required, recrystallised from propan-2-ol to give pure tris (4-fluorophenyl) phosphonium iodide (80%), m.p. 128-130°C. The material is sufficiently pure to suit most purposes. 1H NMR (400.1MHz; CDCl3): δ 1.40 (3H, d, J=28 Hz, CH3), 7.4 (6H, m, Ar-H, ortho), 7.5 (2H, t, Ar-H meta); 19F NMR (400MHz; CDCl3): δ -155 (1F, m, Ar-F); 31P NMR (162MHz; CDCl3): δ -35.86 (1P, m, P); IR (KBr, cm-1): 2860-2980 (CH aliphatic), 1470-1590 (C=C), 1250 (C-P), 1125 (C-F).
Synthesis of methyl triphenyl-phosphonium iodide 4d
A dry 250 ml, three-necked-round bottomed flask equipped with a rubber septum, a magnetic stirrer and reflux condenser was charged with triphenylphosphine (20 g, 76.5 mmol) in (100 mL) of chloroform. Methyl iodide (12.0 g, 6.0 mL, 84 mmol) was added over a 5 minutes period. The reaction mixture was refluxed on a water bath with gentle stirring for 1 h. The reaction is exothermic; care must be taken to ensure that the reaction is not too vigorous, by appropriate cooling. The mixture was transferred to a single rotavapor to leave colourless oil that crystallized on standing. This can, if required, recrystallised from propan-2-ol to give pure methyl triphenylphosphonium iodide (87%), m.p. 180-182 °C. The material is sufficiently pure to suit most purposes. 1H NMR (400.1MHz; CDCl3): δ 3.1 (3H, d, JP-H=13.0 Hz, CH3), 7.7 (15H, m, Ar-H); 13C NMR (376.5MHz; CDCl3): δ 11.5 (d, JPH=57.3, Hz, CH3, 117.78 (C), 118.96 (C), 130.59 (d, JPC =12.1 Hz, CH), 132.94 (d, JPC =10.56 Hz, CH), 134.68 (CH), ; m/z (FAB): 403 [(M+H),+ 4], 278 [M+H-I),+ 11], [(M-I),+ 100], 276 (17), 225 (6), 200 (8), 183 (28); IR (KBr, cm-1): 2860-2980 (CH aliphatic), 1625 (C=C), 1250 (C-P).
Microbiological studies
Antibacterial activity of all the newly synthesized compounds 4b, 6b and 7b was studied using the disc diffusion method. The bacterial strain used was Escherichia Coli, Staphylococcus Aurous, and Pseudomonas.
The compounds were tested at a concentration of 3.5-4.0 µg/mL and were prepared in DMSO. The Petri dishes used for antibacterial screening were incubated at 37 1°C for 24 hrs. The results were compared to Penicillin G, Oxacilline, and Amoxicillin by measuring zone of inhibition in mm using well plate method. These phosphonium salts showed significant activity against all the bacteria tested, Escherichia Coli, Streptococcus pneumonia, Protius, Staphylococcus Aureus and Pseudomonas. The combination of compounds 7b, 6b and 4b with penicillin G gives a synergistic effect. The determinations antibacterial activity is summarized in table-1.
In microbiological study the penicillin derivatives showed significant activity against all the bacteria tested. These drugs showing significant bacterial activity are due to the presence of protective groups.
Table 1: Antibacterial activity concentration – 3.5-4.0 µg/ml
| S Compounds Diameter of zone of inhibition in (mm)
No E.Coli S.Aureus Pseudomonas |
| 1 Pin.G (6.0 µg/mL) – 31.0-38.5 –
2 Oxacillin (5.0 µg/mL) – 27.0-34.0 – 3 Amoxicillin (25.0 µg/mL) 22.0-26.5 – – 4 7b (3.5 µg/mL) 20 – 12 5 Pin.G+7b (3.5 µg/mL v/v) 22 – 13 6 6b (3.5 µg/mL) – 20 – 7 Pin.G+6b(3.5 µg/mL v/v) – 26 – 8 4b (4.0 µg/mL) 10 18 – 9 Pin.G+4b (4.0 µg/mL v/v) 13 22 – |
The combination of compounds 4a, 5b, 6b, 5a and and 4b with penicillin V gives also a synergistic effect for the bacteria S. Aureus, and Sreptococcus. The determinations antibacterial activity are summarized in table-2.
The synthesized compounds were also tested at a concentration of 3.5-4 µg/ml and were prepared in DMSO. The results were compared to penicillin V by measuring zone of inhibition in mm using well plate method.
Table 2: Antibacterial activity concentration – 3.5-4.0 µg/ml
| S Compounds Diameter of zone of inhibition in (mm)
No S.Aureus Sreptococcus |
| 1 Pin.V+4a (160 µg/mL v/v) 24 22
2 Pin.V+5b (3.5 µg/mL v/v) 21 30 3 Pin.V+6b (3.5 µg/mL v/v) 19 28 4 Pin.V+5a (96 µg/mL v/v) 24 22 5 Pin.V+4b (3.5 µg/mL v/v) 20 23 6 Pin.V+7b (4.0 µg/mL v/v) 23 19 7 Pin.V+7a (3.5 µg/mL v/v) 22 20 |
Acknowledgements
The authors are thankful to Dr. alen Brisdon and the staff of analytical and spectroscopic services of the Chemistry Department, University of Manchester for their assistance.
References
- Sekhri, M. L. Kadri, S. Choula and M. Snigra, Biomedical & Pharmacology Journal, 1 (2), 257 (2008).
- Thirupathaiah, Yoshihisha Takaishi and G. Venkateshwar Rao, Biomedical & Pharmacology Journal, 1 (2), 331 (2008).
- S. Jayashree, A.R. Sahu, M. Srinivasa Murthy and K.N. Venugopala, Asian J. Chem., 19 (1), 73 (2007).
- Cete, H. Ozkan, F. Arslan, Y.Yildirir and A. Yasar, Asian J. Chem., 19 (1), 550 (2007).
- Shiradkar, R. Kale, B. Baviskar and R. Dighe, Asian J. Chem., 19 (1), 449 (2007).
- T.Tayade, J. S. Waghmare and S. U. Patile, Asian J. Chem., 19 (1), 443 (2007).
- Algul, N. Duran and G. Gulbol, Asian J. Chem., 19 (4), 3085 (2007).
- Singh, J. Gautam, A. K. Jain and P. K.Mishra, Oriental Journal of Chemistry, 23 (2), 641 (2007).
- Suresh Kumar, R. Rajesh and Annes A. Siddiqui, Oriental Journal of Chemistry, 22(3), 641 (2006).
- Byczynska, I. Gajewski, A Bromiskirski, B. Carlson, R. Kozba, W. Majewska, I. Kups and J. Szkiela, Pol PL 173, 542 (1998).
- Byczynska, I. Gajewski, A Bromiskirski, B. Carlson, R. Kozba, W. Majewska, I. Kups and J. Szkiela, Chem. Abstr., 129, 204 (1998).
- M. A. Nissen, J. Chromatogr. A 812, 53 (1998).
- F. Wishart, J. Am. Vet Med Assoc., 185, 1106 (1984).
- L. Tyczkowska, R. D. Voyksner, A. L. Aronson, J. Chromatogr. 594, 195 (1991).
- M. Siegel, R. K. Isensee, D. J. Beck, Anal. Chem. 59, 989 (1987).
- A. barnes, A. K. Brisdon, F.R.William. Brown, Wendy I Cross, I. R. Crossley, C. Fish, J. V. Morey, R. G. Pritchard, and L. Sekhri, New. J. Chem., 28, 828 (2004).
- F. Khaled, Applied Surface Science 230 (2004) 307-318.
- Sekhri, A. A. Bebba, and Z. Hassini, Asian Journal Chemistry, 17 (4), 2455 (2005).
- S. Frank, S. C. Patrick, J. Chromatogr, A812, 99 (1998).
- R. Mehta, R.R. Shah, S.K. patel and K. C. Patel, Acta Cienc. Indica, 30C, 9 (2004).
- S. Patel, P.S. Patel, K.C. Patel and S. K. Patel, Asian J Chem., 14, 420 (2002).
- Shamsipur, Z. Talebpour, H. R. Bijanzadeh, S. Tabatabaei, Journal of Pharmaceutical and Biomedical Analysis, 30, 1075 (2002).
- L. Tyczkowska, R. D. Voyksner, R. Strauk, A. L. Aronson, J. Chromatogr. 594, 195 (1992).
- Marzo, N. Monti, M. Ripamonti, J. Chromatogr. 507, 235 (1990).







