J. Srivastava, N. Raghava*, R. P. Raghava and L. Singh
Research Lab, FIST-DST Sponsored P.G. Department of Botany, S.M.M.Town (P.G.) College, Ballia - 277 001 India.
Abstract
A pot experiment was performed to observe the effect of Parthenium weed extract on various growth and biochemical parameters of cowpea. The study was conducted to explore the observations, indicated that Parthenium hysterophorus strongly react with Vigna unguiculata (L) Walp. The aqueous extract 20 – 40 % obtained from root of Parthenium plant increased the total chlorophyll and total soluble sugar over control. The toxicity of plant parts extract was also concentration dependent. The leaf extract cause more inhibition than root extract. It was found that allelopathy is an important component of the interaction. The inhibitory potential increased with concentration.
Keywords
Aqueous extract; Parthenium parts; Chlorophyll content; Total soluble sugar
Download this article as:| Copy the following to cite this article: Srivastava J, Raghava N, Raghava R. P, Singh L. Potential use of Parthenium Extract on Growth Parameters, Chlorophyll Content and Total Soluble Sugar of Cowpea. Biomed Pharmacol J 2010;3(2) |
| Copy the following to cite this URL: Srivastava J, Raghava N, Raghava R. P, Singh L. Potential use of Parthenium Extract on Growth Parameters, Chlorophyll Content and Total Soluble Sugar of Cowpea. Biomed Pharmacol J 2010;3(2). Available from: http://biomedpharmajournal.org/?p=1574 |
Introduction
Allelopathy is the new branch of Science and is described as the beneficial and deleterious biochemical interaction between plant and microorganism (Rice, 1984). With the help of allelopathy weed-crop, crop-weed, crop-crop, weed-weed interaction can occurs. Allelopathic effects are both positive and negative and these are utilized for higher crop production. Negative (stimulatory) Allelopathic effect of any weed crop can be utilized to develop eco-friendly, cheap and effective “Green growth promoters”. Similarly, the positive (inhibitory) Allelopathic effect of any weed or crop on weed can utilized to develop “Green herbicides. Many studies conducted at the Department of Agronomy, IGAU, Raipur to know the allelopathic effect of common weeds on growth and germination of many popular agriculture crops. The studies revealed that all weeds are not harmful and the extract of these weeds can be utilized beneficially for crop production.
According to Putnam and Tang (1986) alleged cases of allelopathy that have been studied appear to involve the complex chemical. Rizvi and Rizvi (1992) pointed out that subject not only deals with the biochemical interaction and their effect on the physiological process but also with the mechanism of action of allelochemical at specific site of action at molecular level. Few studies on allelopathy concentrate on the mechanism and processes, involved in the production of allelochemicals, most allelochemical are secondary metabolites and are produced as byproducts of Primary metabolic pathway (Rice, 1984; Putnam, 1985; Putnam and Tang 1986; Rizvi and Rizvi, 1992;Narwal, 1994). They also reported that allelochemicals are present in all plant tissue i.e. leaves, fruits, stems, and Roots. These allelochemicals are released by process of volalization, plant exudation, leaching and decomposition on the plant residue. According to Aldrich (1984) allelochemicals must be concentrated in the leaves, stem or root rather than flower.
As demands increase for sustainable agriculture and concern grow regarding the extensive use of synthetic chemicals. Several workers have shown that allelopathy play important role in weed-weed interaction and weed-crop interaction (Ramaswami, 1997; Khalid, 2000; Park et al., 2003; Jabeen and Ahmed 2009; Srivastava et al., 2010). Parthenium (Parthenium hysterophorus L.) is an exotic weed, accidentally introduced in India in 1955, through the imported food grains (Rao, 1956) but the earliest record of this species goes back to 1814 by William Roxburgh, ‘the father of Indian Botany’ (Paul, 2010). At present, it has occupied almost all parts of India. Apart from its competitive ability, the invasiveness of this noxious weed is thought to be due to an ability to displace other species by means of allelopathy. The allelopathic nature of Parthenium has been well-documented and water-soluble phenolics and sesquiterpene lactones have been reported from roots, stem, leaves, inflorescence, pollen and seeds (Rodriguez, 1975; Kanchan 1975; Towers et al., 1977).
On the basis of review the least work has been observed in leguminous crops, which are rich source of protein in vegetarian diet. The work has been selected to explore the potential use of this allelopathic plant, Parthenium, as positive or stimulatory effect on growth and biochemical parameters of Vigna unguiculata (L) Walp (Cowpea / Lobia; family- Fabaceae) an annual plant.
Materials and Methods
Preparation of extract
The plants of Parthenium were collected from the college campus. The two part of plant (root and leaf) were used for the preparation of extract. The root and leaf samples of this weed were cut into fine pieces immersed in lukewarm water (1.4 mg/ml), kept it for 24 hrs at room temperature. The material was sieved through 2mm mesh, and stock solution of extract was prepared. From this stock solution, different concentration (20,40,80,120,140%) were prepared against control, distilled water (DW) for the spray treatment at different stages of growth (Pre-flowering, flowering and Post-flowering.
Culture technique
The pot experiment was setup in the month of Feb (2008-2010) and harvested in May of same season (average temperature 15° C – 30° C). The soil has pH 6.8, 0.51% organic carbon, 13.5 kg/ hectare phosphate and 240-kg/ hectare potash. The pots were filled with sandy loam (2kg/bag) The soil had pH 6.8 and contain 30.51% organic carbon, 13.5-kg/ hectare phosphate and 240-kg/ hectare potash. According to the soil testing report, the cowdung (200 g/kg soil) was added. The viable seed of cowpea were sown at equal distance in the pot. For each treatment triplicate pots were maintained. The soil of each pot was thoroughly watered. The treatment was given at 3 stages, Pre-flowering, flowering and post flowering (20, 40 and 60 DAS). The observations were recorded at 25, 45 and 65 DAS (Days After Sowing). The growth parameters taken were root and shoot length, number of leaves and leaf area and biochemical parameters like chlorophyll content (Arnon, 1949) at all the three stages of growth and total soluble sugar in seed (Morris, 1948, modified by Highkin and Frenkel, 1962) at final stage of observation.
Statistical analysis
All the data collected on growth, productivity and biochemical attributes were subjected to statistical analysis of variance subjected to randomized block design as described by Panse and Sukhatme (1985).
Results and Discussion
The observation revealed that root extract (RE) and leaf extract (LE) of Parthenium plant, significantly affect different growth parameters and biochemical estimations. These findings showed similarities with the studies of Mersie and Singh (1987), Oudhia (1998, 1999), Singh et al., (2008; 2009; 2010), Maharajan (2006).
The Lower concentrations significantly enhanced the root length at different stages of plant growth. The increase in root length varies from 2.28% to 25.86% under different concentrations at three growth stages (Table-1). The maximum increase in root length was recorded with 40 % RE ie. 22.54 % at 25 DAS, 25.86 % at 45 DAS, and 14.41% at 65 DAS. The data showed that the increase between 45 to 65 DAS is more as compared to the increase in Root length from 25 to 45 DAS. Similarly, the lower concentration of LE significantly enhanced the root length of different stages of plant growth. The increase in root length ranges between 3.54 % to 20.35% under different concentration The maximum increase in root length was recorded with 20 % of leaf extract i.e. 19.93 % at 25 DAS, 20.35 % at 45 DAS, and 15.17 % at 65 DAS. Contrary to present findings, earlier reports suggest that very low concentration (1%) increase the root length (Tefera, 2002), but these observations showed the maximum elongations of root length up to 24.73 cm (control, 21.62 cm) with 40% RE in cowpea and 24.90 cm with 20% LE. Cowpea showed significant enhancement in root growth parameters up to 20 or 40% of PE sprayed at different stages of growth.
The shoot length was also increased with lower concentrations of RE and LE. The maximum increase in shoot length was observed with 40 % RE i.e. 27.26 % at 25 DAS, 12.44% at 45 DAS, and 20.99 % at 65 DAS (Table-1). The higher concentration 140% of root extract showed non-significant increase in shoot length at 45 DAS. The LE is more effective than RE. The maximum increase in shoot length was recorded with 20% LE, i.e. 16.08 % at 25 DAS, 5.15 % at 45 DAS and 17.89 % at 65 DAS. The increase in number of leaves varies from 17.59 % to 34.69 % with different concentrations of RE. The maximum increase in number of leaves was recorded with 40 % RE i.e. 31.88 % at 25 DAS, 34.69 % at 45 DAS and 33.79 % at 65 DAS. But under LE the number of leaves increased with only 20 % concentration. The maximum increase in was observed i.e. 26.37 % at 25 DAS, 31.28 % at 45 DAS and 28.98 % at 65 DAS (Table-2). The earlier studies revealed that even the lower concentrations were inhibitory for leaf growth (Pandey et al., 1993; Bajaj et al., 2004).
Table 1: Effect of different parts of Parthenium extracts on root length (cm) and shoot length (cm) of cowpea, at different stages of plant growth (All the values are an average of nine replicates and three-season crop).
| DAS
Conc. |
25 |
45 |
65 |
25 |
45 |
65 |
|
|
RE Root Length Shoot Length |
|||||||
| CON | 10.20
|
11.74 | 21.62 | 25.42 | 79.11 | 98.92 | |
| 20 | 12.26
|
14.53 | 24.53 | 31.51 | 87.92 | 98.92 | |
| 40 | 12.50
|
14.77 | 24.73 | 32.39 | 88.95 | 119.33 | |
| 80 | 11.76
|
14.46 | 24.30 | 31.16 | 84.84 | 121.67 | |
| 120 | 11.36
|
14.22 | 22.55 | 29.61 | 82.78 | 118.29 | |
| 140 | 10.43*
|
12.85 | 22.71 | 28.73 | 81.07* | 116.97 | |
| CD at 5% level | 0.93
|
1.28 | 2.82 | 1.68 | 2.50 | 2.42 | |
|
LE |
|||||||
| 20 | 12.21
|
14.13 | 24.9 | 29.54 | 83.19 | 116.62 | |
| 40 | 11.86
|
13.95 | 24.60 | 29.22 | 81.85 | 116.18 | |
| 80 | 11.63
|
13.63 | 24.36 | 28.92 | 81.52 | 114.77 | |
| 120 | 11.43
|
13.13 | 22.31 | 28.59 | 80.95* | 113.77 | |
| 140 | 11.03
|
12.9 | 21.03 | 27.08* | 80.95* | 113.22 | |
| CD at 5% level | 0.75 | 1.53 | 2.13 | 1.64 | 1.92 | 2.90 | |
CON: Control., Conc: concentration. *: Non-significant, RE: root extract., LE:leaf extract DAS: Days After Sowing
Table 2: Effect of different parts of Parthenium extracts on number of leaves and leaf area/plant (cm2) of cowpea, at different stages of plant growth(All the value are an average of nine replicates and three seasons crop).
| DAS
Conc. |
25 |
45
|
65
|
25 |
45 |
65 |
|
|
RE Number of Leaves Leaf Area |
|||||||
| CON | 3.03
|
6.83
|
10.74 | 89.98 | 445.64 | 1418.70 | |
| 20 | 3.9
|
8.96 | 14.18 | 107.62 | 528.07 | 1564.69 | |
| 40 | 4.0
|
9.2 | 14.37 | 111.48 | 562.23 | 1593.94 | |
| 80 | 3.66
|
8.86 | 13.81 | 104.62 | 512.63 | 1567.25 | |
| 120 | 3.56
|
8.63 | 13.78 | 102.06 | 459.17* | 1519.31* | |
| 140 | 3.56
|
8.5 | 13.11 | 95.12* | 434.79* | 1478.5* | |
| CD at 5% level | 0.19 | 0.53 | 0.75 | 9.92 | 65.20 | 124.93 | |
|
LE |
|||||||
| 20 | 3.83
|
8.96 | 13.85 | 103.56 | 542.41 | 1531.95 | |
| 40 | 3.73
|
8.73 | 13.66 | 102.89 | 512.45* | 1521.27 | |
| 80 | 3.63
|
8.7 | 13.63 | 101.35 | 506.70* | 1450.63* | |
| 120 | 3.56
|
8.1 | 13.51 | 96.67 | 494.87* | 1407.44* | |
| 140 | 3.36
|
7.23* | 12.99 | 92.3* | 437.92* | 1414.30* | |
| CD at 5% level | 0.27 | 0.63 | 0.60 | 3.38 | 94.14 | 96.59 | |
CON: control.,Conc:concentration. *: Non-significant., RE:root extract., LE:leaf extract DAS: Days After Sowing
The RE showed remarkable increase in leaf area / plant with different concentrations, at all the three stages of growth. The maximum increase in leaf area was recorded with 40% RE, i.e. 23.89 % at 25 DAS, 26.19 % at 45 DAS and 12.35 % at 65 DAS. But LE reduced the leaf area with all the concentration, except 20 % (Table-2). The maximum increase in leaf area was recorded with 20 % LE, i.e. 15.09% at 25 DAS, 24.03 % at 45 DAS and 7.98 % at 65 DAS. The observation on leaf area showed that the drastic increase was recorded with 20, and 40% RE and LE at later stage of growth (65 DAS) in cowpea, but LE showed remarkable reduction in leaf area as comparison to RE. These findings suggest that three cumulative spray treatments of RE, drastically changed or increased the leaf area, while LE in cowpea were inhibitory, showing negative allelopathic effect.
The present observations revealed that the total chlorophyll content was increased with all the concentrations of RE at the initial stages of growth (25 and 45 DAS), while at 65 DAS, only 40% RE significantly enhanced the chlorophyll (Fig.-1). But in case of LE at vegetative and flowering stage all the concentrations were slightly promontory except 120 and 140% at 25 DAS, while at 65 DAS, all the concentrations were inhibitory. The total chlorophyll content in the leaves varied from 22.95 % to 82.07 % under different concentration of RE. 40 % of RE showed the maximum increase i.e. 82.07% at 25 DAS, 76.87% at 45 DAS and 60.67 % at 65 DAS and 20 % LE, i.e. 42.67 % at 25 DAS, 32.83 % at 45 DAS and 44.28 % at 65 DAS. Contrary to these studies, Kumari et al. (1985) showed that chlorophyll content was markedly reduced, when leaf leachates were sprayed directly sprayed on crop plants. The reduction in chlorophyll content was also observed by Suresh and Rai (1987) in tree plants and Jayakumar et al. (1990) in groundnut and corn, under allelochemicals. Similarly, Thakur and Siddiqui (2003) observed that even 10% of Leaf extract of Parthenium was inhibitory for chlorophyll content in mustard. But, in present findings, 20% LE also found to be effective in promoting chlorophyll content in cowpea.
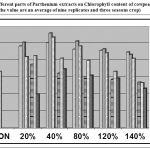 |
Figure 1: Effect of different parts of Parthenium extracts on Chlorophyll content of cowpea, at different stages of plant growth (all the value are an average of nine replicates and three seasons crop).
|
The observation on total soluble sugar in seeds was recorded at the time of maturity. The RE showed better increase in comparison with LE. The present studied also revealed that the total soluble sugar content was also increased with lower concentrations (20 and 40%) of RE, and LE in cowpea, while higher concentrations reduced it. The lower concentration i.e. 40 % of RE showed the maximum increase in total soluble sugar i.e. 26.67 % and the 20% of LE i.e. 17.64 % at maturity (Fig.-2). In relation to sugar content it has been stated that Parthenin (Sesquiterpene lactone) altered this physiological change by reducing the sugar content in Ageratum conyzoides (Singh et al., 2002). Carbohydrates decreased with the passage of time after Parthenium treatment.
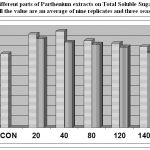 |
Figure 2: Effect of different parts of Parthenium extracts on Total Soluble Sugar of cowpea, at maturity (all the value are an average of nine replicates and three seasons crop).
|
Thus, it is clear that different parts of weed have different allelopathic potential. So, strategies should be made for utilizing allelopathy as an aid in crop production including both avoidance and application protocols. There are immediate opportunities for management of weed and crop residues and minimize crop losses from allelopathy and also to use allelopathic crops for weed management. Allelopathic-environmental interactions must be considered in efforts to benefit from allelopathy. Allelochemicals may also be adapted to yield stimulants or environmentally sound plant growth regulators.
Acknowledgements
The authors’ wishes to thank The Head, Botany Department and The Principal of the college for providing necessary facilities to do the work.
References
- Aldrich, J.D Weed-crop ecology: Principles and Practices. Breton Publishers pp 215-241. (1984).
- Arnon, D.I. Copper enzyme in isolate chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol., 24: 1-15 (1949).
- Bajaj, A., Saxena, M. and Srivastava, S. Allelopathic effects of Parthenium hysterophorus L. on certain foliar parameters of Lantana camara L. In: Narwal, S.S. (Ed) Abstract of the IV Int. Conf. on Allelopathy in Sustainable Terrestrial and Aquatic Ecosystems. Haryana Agricultural University, Hisar, (2004).
- Highkin, H.R and Frenkel, F. Studies of growth and metabolism of a barley mutant lacking Chl b. Plant Physiol. 37:814-820 (1962)..
- Jabeen, N. and Ahmed, M. Possible allelopathic effect of three different weeds on germination and growth of Maize (Zea mays) cultivars. Pak J. Bot., 41(4): 1677-1683 (2009).
- Jayakumar, M., Eyini, M. and Pannerselvan, A. Allelopathic effect of Eucalyptus globulus Labill. in groundnut and corn. Comparative Physiol.Ecol., 15:109-113 (1990).
- Kanchan, S.D. Growth inhibitors from Parthenium hysterophorus Linn. Curr. Sci., 44: 358-359 (1975).
- Khalid, S. Parthenium hysterophorus L. A new introduction to Pakistan. Pak. J. Biol. Sci., 3(5): 846-847 (2000)..
- Kumari, A., Kohli, R.K. and Saxena, D.B. Allelopathic effects of Parthenium hysterophorus leachates and extracts on Brassica campestris L. Annuals of Biology, 1:189-196 (1985).
- Maharajan, S. Phenology, leaf attributes and allelopathic potential of Parthenium hysterophorus L. a highly allergic invasive weed in Katmandu valley. M.Sc. Thesis, Central Department of Botany, Tribhuvan University, Nepal. (2006).
- Morris, D.L. Quantitative determination of Carbohydrates with Drywood’s anthrone reagent. Science, 107:254 – 255 (1948).
- Mersie, W. and Singh, M. Allelopathic effects of Parthenium hysterophorus extract and residue on some agronomic crops and weeds. Journal of Chemical Ecology, 13:1739-1747 (1987).
- Narwal, S.S. Allelopathy in Crop Production, PBI. Scientific Publisher Jodhpur (India) p 288 (1994).
- Oudhia, P. Parthenium: A curse for the biodiversity of Chhatisgarh plants. Abstrect: National Research Seminar on Bio-Chemical Changes. An Impact on Environment, R.D.Govt.P.G.College, Mandla (M.P.) 30-31 july.pp.26,. (1998).
- Oudhia P. Studies on Allelopathy and medicinal weeds in chickpea fields. Chickpea, Pigeon Pea News Letter (ICRISAT), 6: 29-33 (1999).
- Pandey D.K., Kauraw, L.P. and Bhan, V.M. Inhibitory effect of Parthenium hysterophorus residue on growth of water hyacinth (Eichhornia crassipes (Mart.) Solms.) I. Effect of leaf residue. Journal of Chemical Ecology, 19: 2651-2662 (1993).
- Park, S., Laurence, R.B. and Andrew, R.W. The theory and application of plant competition models: an agronomic perspective. Annals of Botany, 92: 741-748 (2003).
- Paul, T.K. The earliest record of Parthenium hysterophorus L. (Asteraceae) in India. Curr. Sci., 98(10): 1272. (2010).
- Putnam, A.R. Weed allelopathy. In: S.O. Duke (ed.) Weed Physiology volume 1: Reproduction and Eco-physiology CRC Press, pp. 131-155 (1985).
- Putnam, A.R. and Tang, C.S., Allelopathy: state of the science IN: A.R. Putnam, & C.S. Tang (ed) The Science of Allelopathy. Wiley, New York 1 – 19 (1986).
- Panse, V.G. and Sukhatme, P.V. Statistical method for agricultural workers. ICAR, New Delhi. (1985).
- Ramaswami, P.P. Potential use of Parthenium. In: Proc. First International Conference on Parthenium Management. Vol .I: 77-80 (1997).
- Rizvi, S.J.H. and Rizvi, V. (Eds.) “Allelopathy: Basic and Applied Aspects”. Chapman and Hall, London. pp.1-8(1992).
- Rao, R.S. Parthenium. A new record for India, J. Bombay Nat. Hist. Soc., 54: 218-220 (1956).
- Rice E.L., Allelopathy 2nd edition. Academic Press, New York (1984).
- Rodriquez, E. Chemistry and distribution of sesquiterpene lactones and flavonoids in Parthenium (Compositae): Systematic and Ecological implications. Ph.D. thesis, University of Texas (1975)..
- Singh, L. Studies on Allelopathic effect of Parthenium on some vegetables. Ph.D. Thesis, VBS Purvanchal University, Jaunpur, India (2010).
- Singh, H.P., Batish, D.R., Kohli, R.K., Saxena, D.B. and Arora, V. Effect on parthenin-A sesquiterpene lactone from Parthenium hysterophorus on early growth and physiology of Ageratum conyzoides. Journal of Chemical Ecology, 28: 2169-2179 (2002).
- Singh, L., Raghava. N., Raghava R.P. and Jayanti Allelopathic effect of Parthenium on Lagenaria siceraria (Mol) Standl. Indian J. Applied and Pure Bio, 23(2): 285-294 (2008).
- Singh, L. Raghava, N. and Raghava, R.P. Allelopathic substances of Parthenium weed and interaction of Solanum melongena. Bioscience, Biotechnology, Research Asia, 6 (2): 855- 862 (2009).
- Srivastava, J., Raghava, N. and Raghava, R.P. Allelopathic potential of Parthenium extract to promote germination of cowpea seeds underwater stress. Biospectra, 5(2): (2010) (In Press).
- Suresh, K. K. and Rai, R.S.V. Studies on the allelopathic effects of some agroforestry tree crops. International Tree Crops Journal, 4: 109-115 (1987).
- Tefera, T. Allelopathic effects of Parthenium hysterophorus extracts on seed germination and seedling growth of Eragrostis tef. Journal Agronomy and Crop Sci., 180: 306-310. (2002).
- Thakur, R. and Siddiqui N. Allelopathic effect of aqueous extract of three common weeds on photosynthetic content of mustard. Indian J. Applied and Pure Bio., 18 (1): 99-102.(2003).
- Towers, G.H.N., Mitchell, J.C., Rodriguez, E., Bennett, F.D. and Subba Rao, P.V. Biology and chemistry of Parthenium hysterophorus L. a problem weeds in India. Journal of Scientific and Industrial Research,. 36: 672-684 (1977).







