Manuscript accepted on :May 15, 2010
Published online on: 21-11-2015
Plagiarism Check: Yes
Raj Narayan Sharma
Department of Chemistry, Shri Mant Madhav Rao Scindia Govt. Model Science College, Jiwaji University, Gwalior - 474 002 India.
Abstract
1-[(N-cinnamoyl) 2, 5-dichloroanilinomalonyl] 3, 5-dimethyl-4-(Unsubstituted/substituted phenylazo) pyrazoles have been synthesized in 38 to 66% yield, by the reaction of 2, 4-diketo-3- (Unsubstituted/substituted phenylazo) pentanes with Ethyl-2-[(N-cinnamoyl) 2, 5-dichloroanilido] acetohydrazide. Pyrazoles are brown and yellow color solids, having high melting points. Identity of products has been established by elemental analysis and spectral data. Newly synthesized compounds [5a-t] have been tested for their antibacterial activity against gram positive bacteria S.albus, S.aureus and gram negative bacteria E.Coli and Pseudomonas piosineus .The compound 5a, 5c, 5d, 5e, 5g and 5h shown significant activity and compound 5b, 5f, 5i, 5j, 5k, 5n and 5p have shown moderate activity. The same compounds were tested for their antifungal activity against Candida albicans, Aspergillus Niger and Alternaria alternata at concentration of 30 mg/ml using sabouraud dextrose agar media. Compounds 5a, 5c, 5d, 5g, 5j, 5m, and 5p were found to be moderately active against Candida albicans and Aspergillus Niger. All the other compounds did not show significant activity against the fungi at the concentration used.
Keywords
Arylazopyrazoles; Synthesis; Characterization and Biological activities
Download this article as:| Copy the following to cite this article: Sharma R. N. Synthesis, characterization and Biological Activities of Some New 1-[(N-cinnamoyl) 2, 5-dichloroanilinomalonyl] 3, 5-dimethyl-4-(unsubstituted/substituted phenylazo) pyrazole Derivatives. Biomed Pharmacol J 2010;3(1) |
| Copy the following to cite this URL: Sharma R. N. Synthesis, characterization and Biological Activities of Some New 1-[(N-cinnamoyl) 2, 5-dichloroanilinomalonyl] 3, 5-dimethyl-4-(unsubstituted/substituted phenylazo) pyrazole Derivatives. Biomed Pharmacol J 2010;3(1). Available from: http://biomedpharmajournal.org/?p=1168 |
Introduction
Pyrazoles and their derivatives are important on account of use in therapy in different diseases1-12 Antibacterial13-20, fungicidal21-27, antidiuretic28-30, anticancer31-37 and anti-HIV38-42 antitumour43, antianalgesic-inflamatory44-48, anticonvulsant49, 50 properties of pyrazoles have been reported in the literature. Synthesis and interesting aspect of biological activity of arylazopyrazoles have been reported51-52. In view of potential biological activities of pyrazoles and arylazopyrazoles we report here in the synthesis of new 1-[(N-cinnamoyl) 2, 5-dichloro anilinomalonyl] 3, 5-dimethyl- 4 – (Unsubstituted/substituted phenylazo) pyrazoles. The present communication deals with the reaction of acetyl acetone with diazotized aromatic primary amine in presence of sodium acetate which furnished 2,4-diketo-3- (Unsubstituted/substituted phenylazo) pentane (I) which on treatment with ethyl-2-[(N-cinnamoyl)2, 5-dichloroanilido] acetohydrazide (II) in acetic acid medium resulted in the formation of 1-[(N-cinnamoyl) 2, 5-dichloroanilinomalonyl] 3, 5-dimethyl-4-(Unsubstituted/substituted phenylazo) pyrazoles (5a-t) in varying yield 38-66% (Table-1). Antibacterial and antifungal activities of new arylazopyrazoles were determined.
Experimental
All the chemicals were used for synthesis are of analytical reagent grade. Melting points are taken in open capillaries and are uncorrected. Purity of the compounds was checked by TLC. All the compounds gave satisfactory elemental analysis. IR Spectra were recorded on a Perkin-Elmer Spectrum RX1 FT IR Spectrophotometer using KBr pallatisation technique and NMR Spectra were recorded on Broker DRX-300 NMR Spectrophotometer. The NMR peaks were recorded on δ scale (ppm) against TMS. The solvent employed was DMSO (3.33-3.35 δ).The elemental analysis of all the compounds done on elemental Vireo EL- III Carlo Erba 1108. 2, 4-Diketo-3- (Unsubstituted/substituted phenylazo) pentane were synthesize by reported method53. Ethyl-2-[(N-cinnamoyl) 2, 5-dichloroanilido] acetohydrazide was prepared by an adaptation of the procedure given by Rathore and Ittyerah54.
Synthesis of Ethyl-2-[2, 5-dichloroanilido] Ethanoate
A mixture of 2, 5-dichloroaniline (10ml) and diethylmalonate (20ml) was refluxed for forty five minutes in a round bottomed flask fitted with an air condenser of such a length (14″) that ethanol formed escaped and diethylmalonate flowed back into the flask. Contents were cooled, ethanol (30 ml) was added, when malon-2,5dichlorodianilide separated out. It was filtered under suction. The filtrate was poured on to crushed ice (Ca160g) and stirred when ethyl-2-(2, 5-dichloroanilido) ethanoate precipitated as green mass. On recrystallization from aqueous ethanol (50%), ester was obtained as white crystals. Yield; 81%, M.P.880C, M.W.276. Anal. Calculation for C11 H11 N1 O3 Cl2 : Found ; C 39.20, N 4.14, Cl 21.5, Calcd. C 39.21, Cl 21.6, N 04.15 IR [KBr] Vmax cm-1 : 1665-1660 [ C=O diketone ], 1290 [ -C-O- Ester], 760-755 [ 2, 5- disubstituted benzene ], 1250 [ C-Cl Stretching ], 1590, 1520 , 1440 [C=C Ring stretching], 3150 [N-H Stretching], 3040[C-H aromatic], 1330-1322[C-H Stretching ]. PMR (DMSO): δ 4.42 (2H, s, CO-CH2-CO), 4.0 (2H, s, NH2), 7.4-8.6 (3H, m, Ar-H), 9.2 (1H, s, CO-NH D2O exchangeable), 10.6 [1 H, s, Ar-NH D2O exchangeable].
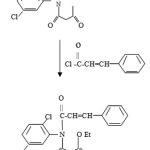
Synthesis of Ethyl-2-[(N- cinnamoyl) 2, 5- dichloroanilido] ethanoate
Cinnamoyl chloride (10gm; 0.06mol), dioxane (6ml), Ethyl-2-(2,5-dichloroanilido) ethanoate (16.5 gm; 0.06 mol) and Triethylamine (6.06 gm; 0.06 mol) were placed in a round bottomed flask carrying reflux condensor having calcium chloride guard tube. The contents were heated on a boiling water bath for three hours and kept over night when triethylamine hydrochloride separated. It was filtered under suction and the filtrate was poured on to crushed ice (Ca180g) and stirred when Ethyl-2-[(N-cinnamoyl) 2, 5-dichloroanilido] ethanoate separated or solid. It was filtered under suction, dried and purified by recrystallisation from aqueous methanol (1:1) in white crystals. Yield = 74 %, MP = 97°C Anal. calculation for C20 H17 N1 O4 Cl2 : [FW = 406 ] , Calculated: N 02.60 , C 43.7, H 03.10 , O 11.7 , Cl 12.9 , Found : N 02.64, C 43.6 , H 03.3 , O 11.5 , Cl 12.8. IR [KBr] Vmax cm-1 : 1740 [ C=O diketone ], 1310 [ -C-O- Ester], 765[ 2, 5- disubstituted benzene ], 1095 [ C-Cl Stretching ], 1590, 1520 , 1440 [C=C Ring stretching ], 3170 [N-H Stretching], 3040[C-H aromatic], 1330-1320 [C-H Stretching]. PMR (DMSO): δ 4.5 [2H, s, CO-CH2-CO], 4.2 [2H, s, NH2], 7.2-8.5 [3H, m, Ar-H], 9.5 [1H, s, CO-NH D2O exchangeable], 10.7 [1H, s, Ar-NH D2O exchangeable].
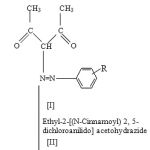
Synthesis of Ethyl-2-[(N- cinnamoyl) 2, 5-dichloroanilido] acetohydrazide
Ethyl-2-[(N- cinnamoyl) 2, 5-dichloroanilido] ethanoate (12.2 gm; 0.03 mol), ethanol (8 ml) and hydrazine hydrate (15 ml; 80%) were mixed together and stirred for forty five minutes. Ethyl-2-[(N-cinnamoyl) 2, 5-dichloroanilido] acetohydrazide was filtered under suction and recrystallised from ethanol in white crystals. Yield; 71%, MP = 184°C, MW 392 Anal. calculation for C18 H15 N3 O3 Cl2 : Calculated ; N 7.6 , C 39.1 , Cl 12.9, Found; N 7.4, C 38.9, Cl 12.7 . IR [KBr] Vmax cm-1: 3170 [N-H Stretching], 3055 [C-H aromatic], 1665 [C=O diketone], 1440 [C-Cl aromatic], 1590, 1530, 1440 [C=C ring stretching]. PMR (DMSO): δ 4.4 (2H, s, CO-CH2-CO), 4.1 (2H, s, NH2), 7.3-8.6 (3H, m, Ar-H), 9.5 (1H, s, CO-NH D2O exchangeable), 10.8 (1H, s, Ar-NH D2O exchangeable).
Synthesis of 2, 4- diketo-3-(phenylazo) pentane (R = H)
Aniline (9.3 ml, 0.1mol) was dissolved in aqueous hydrochloric acid (80 ml, 1:1). The contents were stirred, cooled (0-2˚C) and cold solution of sodium nitrite (12.0 g in 30 ml water) was slowly added maintaining the temperature between 0-2˚C. The cold diazotized solution was added drop wise with stirring to a well cooled mixture of acetyl acetone (0.1 mol, 10 ml) and sodium acetate (12 g dissolved in 10 ml of 50% aqueous ethanol). Stirring was further continued for forty five minutes, when yellow crystals separated. The product was filtered under suction, washed with water and recrystallised from aqueous ethanol.
Analytical [%] for C11H12N2O2: Found; C 38.17, H 03.47, O 9.25, N 08.09, Calcd.; C 38.16, H 03.46, O 9.23 , N 8.00 , Yield; 59 %, M.P.; 96˚C , [ MW 204], Other 2, 4-diketo-3- (Unsubstituted/substituted phenylazo) pentane were prepared by above mentioned procedure.
 |
Scheme 1 |
 |
Scheme 2 |
Synthesis of 1-[(N-cinnamoyl) 2, 5-dichloroanilinomalonyl] 3, 5-dimethyl-4-phenylazo) pyrazoles
2, 4-diketo-3-(phenylazo) pentane (0.204g, 0.001 mol) and ethyl-2-[(N-cinnamoyl) 2, 5-dichloroanilido] acetohydrazide (0.392g, 0.001mol) were dissolved in glacial acetic acid (10ml) and the solution was refluxed for 14 hrs. The resulting solid was purified by repeated washing with acetic acid and recrystallized from acetic acid as yellow crystals. Yield; 57%, M.P.; 262˚C Analysis (%) : Found; N 7.1, Cl 7.2 C29H23N5O3Cl2 [ FW 559] , Calculated; N 7.0, Cl 7.1 IR (KBr) Vmax cm-1: 3270-3060 (N—H Sec. amide hydrogen bond), 2980 (C—H Stretching Aromatic), 1665 (C=N Pyrazole), 1560 (C=C Aromatic), 1060 (C–Cl Aromatic). PMR (DMSO): δ 2.4 (2H, s, CH2), 4.2 (1H, s, NH), 6.90-7.10 S (7H, s, Ar-H). Other 1-[(N-cinnamoyl) 2, 5-dichloroanilinomalonyl] 3, 5-dimethyl-4-((Unsubstituted/substituted phenylazo) pyrazoles were prepared by above mentioned procedure.
Table 1
All compounds gave satisfactory elemental analysis.
| CS. No. | R | Color | M.P. (˚C) | Yield (%) | Molecular Formula | |||||||
| 5a. | H | Yellow | 262 | 57 | C29H23N5O3Cl2 | |||||||
| 5b. | CH3(o) | Light Yellow | 245 | 62 | C30H25N5O3Cl2 | |||||||
| 5c. | CH3(m) | Yellow | 239 | 57 | C30H25N5O3Cl2 | |||||||
| 5d. | CH3(p) | Light Yellow | 237 | 49 | C30H25N5O3Cl2 | |||||||
| 5e. | Cl(o) | Yellow | 268 | 47 | C29H23N5O3Cl3 | |||||||
| 5f. | Cl(m) | Yellow | 264 | 51 | C29H23N5O3Cl3 | |||||||
| 5g. | Cl(p) | Light Yellow | 270 | 53 | C29H23N5O3Cl3 | |||||||
| 5h. | O-CH3(o) | Light Yellow | 265 | 58 | C30H25N5O4Cl2 | |||||||
| 5i. | O-CH3(m) | Yellow | 259 | 52 | C30H25N5O4Cl2 | |||||||
| 5j. | O-CH3(p) | Light Yellow | 267 | 54 | C30H25N5O4Cl2 | |||||||
| 5k. | F(p) | Yellow | 254 | 42 | C29H23N5O3Cl2F1 | |||||||
| 5l. | Br(o) | Dark brown | 251 | 56 | C29H23N5O3Cl2Br | |||||||
| 5m. | O-C2H5 (o) | Brown | 268 | 48 | C31H27N5O4Cl2 | |||||||
| 5n. | O-C2H5 (m) | Brown | 259 | 44 | C31H27N5O4Cl2 | |||||||
| 5o. | O-C2H5 (p) | Brown | 256 | 38 | C31H27N5O4Cl2 | |||||||
| 5p. | CO2H (o) | Brown | 254 | 41 | C30H23N5O5Cl2 | |||||||
| 5q. | CO2H (m) | Brown | 259 | 45 | C30H23N5O5Cl2 | |||||||
| 5r. | CO2H (p) | L. brown | 261 | 42 | C30H23N5O5Cl2 | |||||||
| 5s. | Br(m) | Brown | 249 | 51 | C29H23N5O3Cl2Br | |||||||
| 5t. | Br(p) | Brown | 245 | 53 | C29H23N5O3Cl2Br | |||||||
Biological Activities
Anti-bacterial activity
Newly synthesized compounds (5a-t) have been tested for their anti-bacterial activity against gram-positive bacteria S.albus, S.aureus and gram negative bacteria E.Coli and Pseudomonas piosineus by agar plate disc diffusion method at 30 μg/mL concentrations. Ampicillin and tetracycline were used as a reference compounds. The compound 5a, 5c, 5d, 5e, 5g and 5h shown significant activity and compound 5b, 5f, 5i ,5j, 5k, 5n, and 5p have shown moderate activity.
Anti-fungal activity
The same compounds were tested for their anti-fungal activity against Candida albicans, Aspergillus Niger and Alternaria alternata at concentration of 30 mg/ml using sabouraud dextrose agar media. Compounds 5a, 5c, 5d, 5g, 5j, 5m and 5p were found to be moderately active against Candida albicans and Aspergillus Niger. All the other compounds did not show significant activity against the fungi at the concentration used.
Results and Discussion
1-[(N-cinnamoyl) 2, 5-dichloroanilinomalonyl] 3, 5-dimethyl-4-(Unsubstituted/substituted phenylazo) pyrazoles have been synthesized by the reaction of 2, 4-diketo-3- (Unsubstituted/substituted phenylazo) pentane with Ethyl-2-[(N-cinnamoyl) 2, 5-dichloroanilido] acetohydrazide in 38 to 66% yield, Pyrazoles are brown and yellow color solids, having high melting points. The structure of all the compounds are confirmed by IR, PMR, and Mass spectral data and are further supported by correct elemental analysis (Experimental part). All the newly synthesized compounds (5a-t) have been screened for their antibacterial activity against gram positive bacteria S.albus, S.aureus and gram negative bacteria E.Coli and Pseudomonas piosineus .The compound 5a, 5c, 5d, 5e, 5g and 5h shown significant activity and compound 5b, 5f, 5i, 5j, 5k, 5n, and 5p have shown moderate activity. The same compounds were screened for their antifungal activity against Candida albicans, Aspergillus Niger and Alternaria alternata at concentration of 30 mg/ml using sabouraud dextrose agar media. Compounds 5a, 5c, 5d and 5g were found to be moderately active against Candida albicans and Aspergillus Niger. All the other compounds did not show significant activity against the fungi at the concentration used.
Conclusion
Newly synthesized compounds (5a-t) have been tested for their anti-bacterial activity against gram positive bacteria S.albus, S.aureus and gram negative bacteria E.Coli and Pseudomonas piosineus by agar plate disc diffusion method at 30 μg/mL concentrations. Ampicillin and tetracycline were used as a reference compounds. The compound 5a, 5c, 5d, 5e, 5g and 5h shown significant activity and compound 5b, 5f, 5i, 5j, 5k, 5n and 5p have shown moderate activity. The same compounds were tested for their anti-fungal activity against Candida albicans, Aspergillus Niger and Alternaria alternata at concentration of 30 mg/ml using sabouraud dextrose agar media. Compounds 5a, 5c, 5d and 5g were found to be moderately active against Candida albicans and Aspergillus Niger. All the other compounds did not show significant activity against the fungi at the concentration used.
Acknowledgement
The authors are thankful to principal SMS Government Model Science College, Gwalior for providing research facilities. We are also grateful to Director, C.D.R.I. Lucknow for elemental analysis, and Director, D.R.D.E. Gwalior for spectral studies and Director, Cancer Hospital and Research Institute, Gwalior for Biological activities.
References
- Faraci Willium Stephen;Welch Willard Mckowan, Pct. Int. Appl. W094, 13, 643 (C1.CO7D 231/44) 23 Jun (1944).
- Yasunobukai, Akhiko Tsureka Jpn Kokai Tokovo Koho JP 08 183787 96, 183787 (C1.CO7D 471/04) 10 Jul (1996).
- El. Shaaer H.M., Mansoura Sci. Bull; A; Chem. 24 (Suppl 1), 171-185 (1997).
- Savelli Francesco, Boida Alessandro Farmaco, 51 (2) 141-3 (1996).
- Manfred Manzer; Hans Joachim Lankau, Unverferth Klaus, Ger. Offen. DE 19,521322 (C1.COD 231/38) 19 Dec (1996).
- Chandra Prakash, Saharia G. S. and Sharma H. R., J. Ind. Chem. Soc. 54, 381 (1977).
- S.A. El-Hawash, N.S. Habib and M. Kassem, Arch. Pharm. Chem. Life Sci, 339, 564-571, (2006).
- Amal M. Youssef, Mona H. Badr and Rusha Y. Elbayaa 68th International Congress of FIP, Basel, Switzerland ,29 Augest -4 sept., p-226,(2008).
- Salgin-Goksen U., Gokhan-Kelekci N., Goktas O., Koysal Y., Kihc E., Isik S, Aktay G., Ozalp M., Bioorg. Med.Chem. 15[17], 5738-5751, (2007).
- Kumar A.; Bansal D.; Bajaj K.; Sharma S. Archana Shrivastava V. K..; Bioorg. Med. Chem.11, 5281-91, (2003).
- Vinod Dhingra;Renu Bhatnadekar and Swati Perdse; Indian Journal of Chem. 5[3] 515-518 (1993).
- Godaginamath, G.S., Pujar,S.R., Kavali, R.R. Indian J.Chem., 42[B],2023, (2003).
- Saharia G. S. and. Sharma H. R, Indian Journal of Chemistry, 148, 626 (1976).
- Godaginamath,G.S. ; Pujar,S.R.; Bhovi ,M.G. ; Kamanavalli,C.M. ; Indian J. Heterocycl. Chem. 13, 209, (2004).
- M.A. Abdallah, S.M. Riyadh, I.M. Abbas and S.M.Gomha, J. Chinese Chemical Soc. 52, 987-994,( 2005).
- Bekhit A.A.; Abdel-Aziem T. ; Bioorg. Med. Chem. 12, 1935-45, 2004.
- Kaymakcioglu B.K.; Rollas S.; Korcegez E.; Aricioglu F. ; Eur. J. Pharm. Sci. (2005). [in press].
- Garg H.G. and. Singh P.P., J. Med. Chem.,11, 1103 (1968).
- Saharia G. S. and Sharma H. R., J. Indian Chem. Soc., 21, 137 (1962).
- Rostom S.A., Bioorg Med Chem., 1, 14 (19) 6475-85 (2006).
- Elgandi M.H.E., Elamoghayer M.R.H.,Hafer E.A.A.,and Alniwa H.H., J. Org. Chem., 40, 2604 (1975).
- S. Rollas, N. Gulerman and H. Erdeniz, Farmaco 57, 171-174, (2002).
- Nadia Adil SALIH; Turk J. Chem. 32, 229-235,( 2008).
- R.A. Devasia, T.F. Jones, J.Ward, L. Stafford, H.Hardin, C.Bopp, M.Beatty, E. Mintz, W. Schaffner, Am. J. Med., 119, 168-176, (2006).
- Shaheen H.I., Khalil S.B., Rao M.R., Elyazeed R.A., Wierzba T.F., Peruski L.F., Putnam S., Navarro A., Morsy B.Z., Cravioto A., Clemens J.D., Svennerholm A.M., Savarino S.J., Egypt, J. Clin. Microbiol., 42, 5588-5595, (2004).
- Metwally K.A., Abdel-Aziz L.M., Lashine el- S.M., Husseiny M.I., Badawy R.H., Bioorg. Med. Chem., 14 [24], 8675-8682, (2006).
- Vicini P., Zani F., Cozzini P., Doytchinova I., Eur. J. Chem. 37, [7] , 553-564, (2002).
- Garg H.G. and. Singh P.P., J. Med. Chem., 11, 1103 (1968)
- Farghaly A.A., Bekhit A.A., Park J.Y., Arch. Pharm., 333[2-3], 53-57, (2000).
- Wang R., Lu X., Yu X., Shi L., Sun Y., J. Mol. Cat. A: Chemical, 266[2], 198-201, (2007).
- Saharia G. S. and Sharma H. R., J. Indian Chem. Soc., 21, 137 (1962).
- Rostom S.A., Bioorg Med Chem., 1, 14 (19) 6475-85 (2006).
- Shivarama Holla B, Sooryanarayana Rao B, Sarojini BK., Akberali PM., Suchetha Kumari N., Eur.J. Med. Chem., 41[5], 657-663,(2006).
- T.S. Jeong, K.S. Kim, J.R. Kim, K.H. Cho, S. Lee and W.S.Lee, Bioorg. Med. Chem. Lett. 14, 2719-2723, (2004). DOI: 10.1016/j.bmcl.2004.03.072
- R.Lin., G.Chiu,Y.Yu,P.J. Connolly, S.Li, Y. Lu, M. Adams, A.R. Fuentes –Pesquera, S.L. Emanuel and L.M. Greenberger, Bioorg.Med.Chem. Lett.17,( 2007) , 4557-4561. DOI: 10.1016/j.bmcl.(2007).05.092.
- Holla,B.S., Sarojini B.K.; Rao,B.S; Akberali, P.M. ; Kumari,N.S., Shetty,V.; Farmaco 56, 565, (2001).
- Ibrahim Chaaban, El-Sayeda EL-Khawass , Mona Mohran , Ola EL-Sayed, Hassan EL-Saidi and Hassan Aboul-Enen. Med. Chem. Res. Vol. 15, (2007).
- V.S.Jolly,Manish Pathak and Ragini Jain; Indian Journal of Chem; 32B,505-507 (1993).
- Vinod Dhingra;Renu Bhatnadekar and Swati Perdse; Indian Journal of Chem. 5[3] 515-518 (1993).
- Anjali Gupta; Ph.D.Thesis, Jiwaji University, Gwalior,[M.P.] India (1997).
- Rida S.M., Ashour F.A., El-Hawash S.A., Elsemary M.M., Badr M.H.,Shalaby M.A., Eur. J. Med.Chem. 40, [9], 949-959, (2005).
- Nadia Adil SALIH; Turk J. Chem. 32, 229-235, (2008).
- Salgin-Goksen U., Gokhan-Kelekci N., Goktas O., Koysal Y., Kihc E., Isik S, Aktay G., Ozalp M., Bioorg. Med. Chem. 15[17],5738-5751 , (2007).
- Gulcan H.O., Kupeli E., Unlu S., Yesilada E., Sahin M.F., Arch. Pharm. , 336, 477-482, (2003).
- Godaginamath,G.S. ; Pujar,S.R.; Kavali,R.R.,; Indian J. Chem. 42[b], 2023, (2003).
- Godaginamath,G.S. ; Donawade,D.S. Indian J. Chem. 42[b], 3108, (2003).
- Kumar A.; Bansal D.; Bajaj K.; Sharma S. Archana Shrivastava V. K..; Bioorg. Med. Chem. 11, 5281-91, (2003).
- Tosun A. U., Geciken A.E., Goke M., Yildirim E. Sahin M.F., Turk J. Pharm . Sci. 5[3], 155-166, (2008).
- Onkol T., Sahin M.F., Yildirim E., Erol K., Ito S., Arch. Pharm Res. 27, 1068-1092, (2004).
- Amal M. Youssef, Mona H. Badr and Rusha Y. Elbayaa 68th International Congress of FIP, Basel, Switzerland ,29 Augest -4 sept., p-226,(2008).
- V.K.Ahluwalia; U.Dutta and H.R.Sharma ; J. Indian Chem. Soc., 64, 221 (1987).
- Sharma K. P. and Harendra K. Sharma, Asian J. Chemistry, 19 (5) 4129-4131 (2007).
- Poornima Phatak, Ph.D. Thesis, Jiwaji University, Gwalior (M. P.) India, (1996).
- Rathore B.S. and Ittyerah P.I., J. Indian Chem. Soc., 37, 591(1960).







