J. Myla¹, R. Chandrasekaran² and R. Saravana Muthu³
¹Department of Microbiology, A.V.C. College (Autonomous), Mannampandal, Mayiladuthurai - 609 305 India.
²Department of Botany and Microbiology, A.V.V.M. Sripushpam College, Poondi, Thanjavur - 613 503 India.
³Department of Botany, A.V.C. college (Autonomous), Mannampandal, Mayiladuthurai - 609 305 India.
Abstract
Biosurfactants are surface active compounds produced by microorganisms. These molecules reduce surface tension between aqueous solutions and hydrocarbon mixtures. Oil contaminated soil were collected from different automobile shop. Bacillus and Pseudomonas sp were isolated from the four soil samples and these isolates were screened for biosurfactans activity using petrol, diesel and kerosene source by emulsification capacity, Surface tension measurement, Oil spreading techniques, Drop collapse method and Haemolytic activity. Biosurfactant production by the isolated bacterium using different pH, temperature and concentration of carbon source was studied. Effective production of biosurfactant in both Bacillus and Pseudomonas sp using mineral salt broth with optimiazed condition.
Keywords
Biosurfactant; Oil spreading technique; Haemolytic activity; E24 Index
Download this article as:| Copy the following to cite this article: Myla J, Chandrasekaran R, Muthu R. S. Screening Optimization and Production of Biosurfactants from Bacillus and Pseudomonas sp. Biomed Pharmacol J 2010;3(1) |
| Copy the following to cite this URL: Myla J, Chandrasekaran R, Muthu R. S. Screening Optimization and Production of Biosurfactants from Bacillus and Pseudomonas sp. Biomed Pharmacol J 2010;3(1). Available from: http://biomedpharmajournal.org/?p=1283 |
Introduction
Biosurfactants are amphiphilic compounds produced on living surfaces, mostly microbial cell surfaces or excreted extracellularly and contain hydrophobic and hydrophilic moieties that reduce surface tension and interfacial tension between individual molecules at the surface and interface respectively.(Karanth et al., 1999) biosurfactant producing microorganisms were naturally present in oil contaminated soil. Oil contaminated environment contain large amount of hydrocarbons. i.e., aliphatic and aromatic hydrocarbons. Microorganisms exhibit emulsifying activity by producing biosurfactants and utilize the hydrocarbons as substrate often mineralizing them or converting them into harmless products.
Biosurfactants have gained more importance in the fields of enhanced oil recovery, environmental bioremediation, food processing, and pharmaceuticals owing to their unique properties- higher biodegradability, lower toxicity, and effectiveness at extremes of temperature, pH and salinity. Biosurfactants can be produced by microbial fermentation processes using cheaper agrobased substrates and waste materials.In various industrial processes, they are potencially useful surface-active agents for emulsion polymerization, wetting, foaming, phase dispersionl, emulsification and de-emulsification.(Desai et al., 1997)
Biosurfactants are mainly categorized mainly by their chemical composition and microbial origin. Generally their structures include a hydrophilic moiety consisting of aminoacids or peptides, mono-di or polysaccharides and hydrophobic moiety comprising unsaturated or saturate fatty acids.Accordingly, the major classes of biosurfactants include glycolipids, lipopeptides, lipoproteins, phospholipid, fatty acids, polymeric biosurfactant and particulate biosurfactants.(Manneerat et al., 2005)
Among the different classes of biosurfactants rhamnolipid and surfactin are best studied. Rhamnolipid is produced by Pseudomonas aeruginosa, a gram-negative, motile, non spore forming bacteria. Surfactin is cyclic lipopeptide commonly used as an antibiotic. Surfactin’s structure consists of a peptide loop of seven aminoacids(L-asparagine, glycine, two L-leucine, L-valine, and two D-Leucins) and an hydrophobic fatty acid chain thirteen to fifteen carbon long.(Peypoux et al., 1999)
Materials and Methods
The soil samples were collected from polluted sites and isolate the bacteria Bacillus and Pseudomonas from oil contaminated soil by gram staining and by various biochemical test. Screening of biosurfactants using the following methods.
Screening for biosurfactant activity
The supernatant was subsequently subjected to the preliminary screenig methods by using 3 different oil namly petrol, Diesel and kerosene.
Oil spreading techniques
The 50ml of distilled water was added to a large petri dish (15cm diameter) followed by the addition of 20ml of Oil to the surface of water, 10ml of supernatant of culture broth (Rodrigues et al., 2006)
Emulsification cabacity(E24) test
E24 of culture samples was determined by adding 2ml of oil to the same amount of culture, mixing with a vortex for a 2min and leaving to stand for 24 hours. The E24 index is given as percentage of height of emulsified layer(cm) divided by total height of the liquid column(cm) (Sarubbo 2006)
Table 1: (A) Emulsification Index In Petrol
| Organisms | EmulsifiedLayer (cm) | Total Liquid Column(cm) | % of E24 |
| Bacillus subtilisPseudomonas aeruginosa | 0.60.8 | 2.92.7 | 2130 |
Drop collapse method
For drop collapse method 2ml of oil was added to each well of a 96 well containing microtiter plate lid. The lid was equilibrated for 1 h at room temperature. MSM cultures of bacterial isolates was centrifuged and then 5ml of the drop on the surface of oil was evaluated after 1 min.(Jain et al., 1991)
Table 1: (B)Emulsification Index In Diesel
|
Organisms |
Emulsified Layer (cm) |
Total Liquid Column(cm) |
% of E24 |
|
Bacillus subtilis Pseudomonas aeruginosa |
0.3 0.5 |
3.2 3.0 |
9 17
|
Haemolytic activity
Isolates were screened on blood agar plates containing 5% sheep blood and incubated at 37°C for 48 h. Hemolytic activity was detected as the presence of clear zone around bacterial isolates (Plaza et al ., 2006)
Table 1: (C)Emulsification Index In Kerosine
|
Organisms |
Emulsified Layer (cm) |
Total Liquid Column(cm) |
% of E24 |
|
Bacillus subtilis Pseudomonas aeruginosa |
0.3 0.4 |
3.2 3.1 |
9 13
|
Surface tension measurement
Surface tension of biosurfactant was measured by drop weight method . A burette is held vertically and liquid drops are allowed to from its lower end at a very solw rate about 8 drops per minute. A clean dry beaker is weighted and placed under flow of burette. 50 drops of the liquid are collected and weighted the beaker. Again repeat the same for 3 times.the average mass of one drop is calculated and then surface tension of liquid containg biosurfactant calculated by ( surface tension(T) = Mg/3.8r )
Process optimization and biosurfactant production
The pH ranges from 6,7,and 8, temperature ranges from 35°C, 37°C and 39°C and kerosene concentration from 1%, 3% and 5% for optimization of biosurfactants production in bacillus and pseudomona sp. Both the organisms have maximum growth rate observed at pH 7, temperature of 37°C in 0.3% kerosene concentration. Effective biosurfactants production from Bacillus and Pseudomonas sp can be achieved using Mineral salt broth.
Result and Discussion
Four isolates of bacillus and pseudomonas have highest E24 value was observed in diesel (Priya and usharani 2009). In case our study Bacillus and Pseudomonas have the ability to emulsifying oils. The highest E24 value was observed in petrol and Pseudomonas showed the better E24 value than bacillus. (table –1). In oil spreading techniques bacillus shows higher zone formation of 4.1mm, 3.6mm, 3.2mm in petrol , diesel, and kerosene respectively. Similarly our study focused Pseudomonas showed 3.9mm , 3.3mm, 2.8mm in petrol, diesel, and kerosene.
Surface tension reduction was measured by Kruss Hamburg Nr2215 Tensio meter. Results were compared to medium composition as negative control ( Pavitran et al., 2004) Since our study of surface tension reduction was measured by drop weight method and Pseudomonas shows 0.007 surface tension reduction per drop.
Blood agar method is often used for a preliminary screening of microorganisms for the ability to produce biosurfactants on hydrophilic media. (Schulz et al ., 1991). From our results both the isolates will cause lysis of the blood cells and exhibit a colorless, transparent ring around the colonies.
Optimization of biosurfactants was carried out by using different pH , temperature and concentration of carbon source ( kerosene) (chart-1)
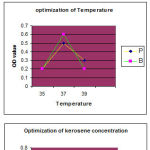 |
Figure 1 |
Conclusion
From the above observation, it was concluded that both bacterial isolates of bacillus and pseudomonas have the ability to secrete surface active agents it is gain more importane in future for industrial and environmental applications.
References
- Desai, J.D., and Banat, I.M., 1997. Microbial production of surfactants and their commercial potencial. Microbiol. Mol. Biol., 61: 47-64.
- Jain, D.K., Collins Thommpson, D.L., Lee, H. 1991. A drop collapsing test for Screening biosurfactant producing microorganisms. J. Microbiol Methods., 13: 271-279.
- Karanth, N.G.K., Deo, P.G. and Veenanadig, N.K. 1999. Microbial Production of biosurfactants and their importance. Curr.Sci., 77: 116-123.
- Maneerat, S. 2005. Biosurfactants from marine microorganisms. Songklanakarin J Sci Technol., 27: 1263-1272
- Peypoux, F., Bonmatin, J.M., and Wallach, J. 1999. Recent trends in Biochemistry Of Surfactin. Applied Microbiol Biotechnol., 51: 553-563.
- Plaza, G.A., Zjawiony, I., Banat, I.M. 2006. Use of different methods for detection of thermophilic biosurfactant producing bacteria from hydrocarbon-contaminated and bioremediated soils. J. Petroleum Sci Engg., 50: 71-77.
- Priya, T., Usharani, G. 2009. Comparative Study for Biosurfactant Production by Using Bacillus subtilis and Pseudomonas aeruginosa. Bot. Res. Intl., 2(4): 284-287.
- Rodrigues, L.R., Teixeira, J.A., Mei, H.C., and Oliveira, R. 2006. Physiochemical and functional characterisation of a Biosurfactant produced by Lactococcus lactis 53, Colloids and surfaces. B: Biointerfaces., 49: 79-86
- Schulz, D., Passeri, A., Schmidt, M. 1991. Screening for biosurfactants among crude-oil degrading marine microorganisms from the North-sea. z Naturforsch ( C )., 46(4): 197-203.
- Saruboo, L.A. 2006. Production and Stability studies of the Bioemulsifier obtained from a strain of Candida glabrata UCP1002. Journal of Biotechnology., 9(4): 400-401.







