Pavani Anumukonda* and Prabhakar Tadimalla
1Pharmaceutical Biotechnology Division, Department of Pharmaceutical Sciences, Andhra University, Visakhapatnam - 530 003 India.
Abstract
Fungi are used in many industrial processes, such as the production of enzymes, vitamins, polysaccharides, polyhydric alcohols, pigments, lipids, and glycol-lipids. Some of these products are produced commercially while others are potentially valuable in biotechnology. Fungal secondary metabolites are extremely important to our health and nutrition and have tremendous economic impact. The screening and isolation procedures resulted, five potentially interesting fungal isolates (MF S2-02, MF S3-09, MF S4-11, MF S5-08, and MF S6-09). All were able to produce more then 50 U/gm DCW of intracellular activity and 10 U /ml extracellular activity. The results indicate that the isolate MF S4-11 is promising strain with the yield of 117 U/gm DCW. A good number of media that were reported in the literature were screened to select optimal production media and medium number ‘IV’ was found to be the best for the production of â-galactosidase (128 U/ gm DCW).
Keywords
b-galactosidase; lactose hydrolysis; transgalactosylation; marine samples
Download this article as:| Copy the following to cite this article: Anumukonda P, Tadimalla P. Screening of B-Galactosidase Producing Fungi from Marine Samples. Biomed Pharmacol J 2010;3(1) |
| Copy the following to cite this URL: Anumukonda P, Tadimalla P. Screening of B-Galactosidase Producing Fungi from Marine Samples. Biomed Pharmacol J 2010;3(1). Available from: http://biomedpharmajournal.org/?p=1162 |
Introduction
The ocean remains as an unexploited source for many drugs and pharmacologically active substances. K Kathiresan1 et al (2008) isolated marine lactic acid bacteria. Pseudomonas BAL-31 is a marine Pseudomonas first isolated from samples of seawater taken from the Pacific Ocean by Espejo and Canelo2 (1977).
No reports are available concerning the presence of β-galactosidase producing fungi from marine environment. Marine environment may yield strains which may produce novel metabolites or potent producers of known metabolites.
β-galactosidase, is the enzyme widely used especially in dairy & food technologies. This enzyme provides two benefits, which make its use attractive in industries: 1) Preparation of lactose-free milk 2) Biosynthesis of Galacto-oligosaccharides that are interesting from the technological as well as health perspective. Low activity of β-galactosidase causes digestive insufficiency, called lactose intolerance. Decreased activity of β-galactosidase is rather frequent especially with seniors. Lactose-free milk is manufactured for those patients (Suarez3 et al. 1995). β-galactosidases are widely distributed in all organisms, but their physiological function is not always fully recognized. In some cases β-galactosidase are able to catalyze trans-galactosylation. It belongs to the glycosidases that shows both hydrolysation and transgalactosylation activity, i.e. they are able to synthesize oligosaccharides during hydrolysis of natural substrates. It was found that two models viz, transglycosylation and reverse hydrolysis (Rastall4 & Bucke 1992) could be used for oligosaccharide synthesis. Oligosaccharides, synthesized by enzymes, are mainly used as food additives with activity to improve physicochemical characteristics (Crittenden5 & Playne 1996). Very few reports are available in the literature on the production of extra cellular and intracellular β-galactosidase from fungi. There are no reports on the production of β-galactosidase from marine fungi. In view of this, it is proposed to isolate and screen marine fungi for the detection of β-galactosidase producing fungi.
Material and Methods
Chemicals
2-nitrophenyl- β-D-galactopyranoside was procured from Carbosynth Limited, Berkshire, RG20 8RY, U.K. ONPG discs (2-nitrophenyl- β-D-galactopyranoside) were purchased from sigma Aldrich (Fluka) and ortho nitro phenol was obtained from Merck (Germany). All other chemicals and medium constituents in this study were of analytical grade and procured from Sigma-Aldrich, Merck and s.d fine chemicals.
Samples
Seven marine samples were collected from Bay of Bengal. Out of seven samples, two were marine sediment samples, two were marine water samples and remaining three were marine sand samples. The sediments were collected with the core sampler near Vishakhapatnam (one at R.K beach and other at Gangavaram beach). These were brought to the lab and processed immediately.
Table 1.1 Distribution of fungi in various marine samples.
| Sample code | Type of sample | Place of collection | Total no of fungi |
| S1 | Marine soil sample | R.K beach | 9 |
| S2 | Marine soil sample | Vuda park | 8 |
| S3 | Marine soil sample | Steel plant | 10 |
| S4 | Marine Sediment | 100 km away from Offshore | 11 |
| S5 | Sea water | Gangavaram | 12 |
| S6 | Marine Sediment | Rishikonda | 13 |
| S7 | Sea water | Theneti park | 9 |
Isolation of fungi
About 1 gm of each sample was transferred in to 50 ml sterile marine water (250 ml EM flask), agitated on rotary shaker for one hour to get uniform suspension and serially diluted. One ml of each appropriate dilution was plated (6 inch diameter plates) on to 50 ml molten PDA and YEME media, and allowed to set and incubated at 300 C for 7 days. Both PDA and YEME dehydrated media were procured from Himedia.
Pure and selected colonies were transferred on to PDA slants and incubated at 300 C for 7 days. The cultures were preserved at 40 C and sub-cultured monthly.
About 72 fungal colonies in total were isolated from the above samples. The isolates were pooled together and the cultures which appear identical to the naked eye with respective colour of the aerial mycelium, reverse mycelium colour, soluble pigments and surface texture were eliminated. 54 different fungal colonies were selected from total 72 colonies.
Screening of the marine fungal isolates for β-galactosidase activity.
The selected 54 isolates were tested for their ability to grow on PDA medium containing lactose as a sole source of carbon. Plates were incubated at 280 C for 5 days. The isolates were inoculated, incubated at 280 C for 5 days and 22 isolates were selected based on the extent of growth. The selected 22 isolates were initially screened for the β-galactosidase producing capacity by using ONPG dicks. Each isolate was plated on to PDA medium and incubated at 280 C for 5 days. Colonies from each plate were picked up and transferred to a test tube of (0.1 ml) sterile saline solution. The sterile ONPG disk (Sigma) was transferred aseptically to the above saline solution. They were incubated at 280 C for 24 hr. The colonies producing β-Galactosidase turned the solution to yellow colour by releasing Ortho-nitro phenol (ONP). The intensity of the colour depends up on ability of the isolate to produce β-galactosidase. Out of 22 fungal isolates, 9 isolates were not able to release ONP and they were considered to be non-producers and 13 isolates were found to be producers of the enzyme. Among these 13, five potent isolates were selected on the basis of the intensity of yellow colour.
Table: 1.2 β-galactosidase production capacity of marine fungi
| S. No | Isolate No. | ONP intensity |
| 1 | MF S1-01 | ++ |
| 2 | MF S1-05 | + |
| 3 | MF S1-09 | – |
| 4 | MF S2-02 | +++ |
| 5 | MF S2-04 | – |
| 6 | MF S2-08 | – |
| 7 | MF S3-03 | + |
| 8 | MF S3-08 | + |
| 9 | MF S3-09 | +++ |
| 10 | MF S4-01 | – |
| 11 | MF S4-05 | + |
| 12 | MF S4-09 | – |
| 13 | MF S4-11 | +++ |
| 14 | MF S5-06 | – |
| 15 | MF S5-08 | +++ |
| 16 | MF S5-10 | – |
| 17 | MF S5-12 | – |
| 18 | MF S6-09 | +++ |
| 19 | MF S6-11 | ++ |
| 20 | MF S6-13 | – |
| 21 | MF S7-02 | + |
| 22 | MF S7-09 | ++ |
– : No β-Galactosidase production
+ : Poor β-Galactosidase production
++ : Moderate β-Galactosidase production
+++ : Good β-Galactosidase production
The results of these 22 isolates are presented in Table 1.2. The selected five isolates MF S2-02, MF S3-09, MF S4-11, MF S5-08, and MF S6-09 were further tested for their β-Galactosidase producing capabilities, by submerged fermentation process.
Production of β-galactosidase by submerged fermentation.
Micro-organisms
All the five promising isolates were grown on a PDA slants with lactose as a carbon source for 7 days at 280 C.
Submerged fermentation
Spore suspension Preparation
From the full grown slant the spores were suspended in 10 ml of distilled water containing 0.01% Triton X-100.
Inoculum preparation
Five ml of sterile distilled water was added to the above full grown slant, and growth content was scraped out and transferred in to 250ml Erlenmeyer flask containing 50 ml of sterile inoculum medium. The composition of the inoculum medium is (g/L): Lactose, 10.0; peptone, 10.0; Yeast Extract, 10.0; Dextrose, 20.0; (NH4) 2 SO4, 5.0; KH 2 PO4, 1.0; K2HPO4 3.0; MgSO4.7H 2O, 0.5. The flasks were incubated on a rotary shaker (150 rpm) at 280 C for 2 days.
At the end of the incubation the cell mass was collected by centrifuging the medium in a refrigerated centrifuge (40 C) at 3000 rpm for 20 minutes. The cell mass was washed twice with sterile distilled water, suspended in 10 ml of sterile distilled water and used as inoculum.
Production of β–galactosidase
The inoculum (10% level) was transferred aseptically to 100 ml production medium contained in 500 ml EM flask. The composition of the production medium is same as above except dextrose. The flasks were incubated on a rotary shaker (150 rpm) at 280 C for 5 days.
The samples (5 ml) were withdrawn for every 24 hr and centrifuged at 1500 rpm for 15 minutes. The clear supernatant was separated and the cell mass was used for the detection of intracellular enzyme activity. Cell free supernatant (CFS) was used for the detection of extracellular enzyme activity. About 0.5 gm of cell mass was used to determine the intracellular enzyme activity. The enzyme assay was carried out as described below.
β-Galactosidase Assay
β-Galactosidase was determined by measuring the release of o-nitro phenol from ONPG (o-nitro-phenyl- β-D-galactopyranoside) at 420 nm.
For intracellular activity 0.5 gm of cells were treated with 0.5 ml of toluene at 370 C for 5 minutes. 2 ml of ONPG (4mg/ml) prepared in citrate buffer (pH 4.6) was added and incubated at 400 C for 15 minutes. The reaction was stopped by the addition of 2.5 ml 10% (w/v) Na2CO3. The release of O-nitro phenol from ONPG was measured at 420 nm with the help of spectrophotometer (UV-VTS Spectrometer 117 from Systronics).
Similarly extracellular enzyme activity was measured using 1ml aliquots of cell free supernatant (CFS).
The results of intracellular and extracellular activities of the selected isolates are presented in the Table 1.3.
Table 1.3: β-Galactosidase producing of marine fungal isolates.
| S.NO | Isolate Number | β-Galactosidase activity | |
| Intracellular (U/gm) | Extracellular (U/L) | ||
| 1 | MF S2-02 | 62 | 10 |
| 2 | MF S3-09 | 79 | 23 |
| 3 | MF S4-11 | 117 | 39 |
| 4 | MF S5-08 | 40 | 33 |
| 5 | MF S6-09 | 52 | 11 |
Among the five isolates MF S4-11 exhibited the highest activity. It had both the intercellular and extracellular activity. The intercellular activity is three times more than the extracellular activity.
Determination of MDW
For the determination of mycelial dry weight, 10 ml aliquots of the sample was with drawn from the medium, Centrifuged the cells for 15 minutes. Decant the supernatant liquid and washed the cells with sterile distilled water two times to remove the medium components. Carefully transferred the cells in to pre weighed aluminum foil. These cells were dried to a constant weight at 800 C. Data were averaged with three trails.
pH profile of the isolate MF S4-11
pH was measured for all the 24 hr samples up to the 5 days. No considerable change was observed.
Enzyme activity: The isolate MF S4-11 showed maximum enzyme activity at 110 hr.
Table 1.4: Composition of different media used for the production of β-Galactosidase
| Medium No | Composition (g/100 ml) | Reference | Enzyme Yield (U/gm DCW) |
| I. | 2.5 gm lactose, 0.4gm (NH4)2SO4, 0.2 gm KH2PO4, 0.03 gm MgSO4.7H2O, 0.015 gm CaCI2 2H2O, 0.02 ml Tween 80, 0.5 mg FeSO4.7H20, 0.16 mg MnSO4H20, 0.14 mg ZnSO47H2O and 0.2 mg COC12.6H20. pH 5.0. | Francisco6 Castillo*, 1984 | 102 |
| II. | 0.6 gm Lactose, 2 gm Soluble Starch, 1 gm Yeast extract, 0.1 gm MgSO4 and 0.1 gm K2HPO4. pH 6.8. | Lutz Fischer7 et al., 1995. | 95 |
| III. | 2 gm Lactose, 1 gm Yeast extract, 0.1 gm MgSO4 and 0.1 gm K2HPO4. pH 5.0 to 5.5. | Nakkharat8. et al., 2005. | 117 |
| IV. | 2 gm Lactose, Peptone 1 gm, Yeast extract 1 gm, glucose 1 gm, (NH4)2SO4 0.5 gm, K2HPO4 0.3gm, KH2PO4 0.1 gm and MgSO4 0.05 gm. pH 6.0. | Onishi9 and Tanaka 1997. | 128 |
| V. | 1 gm lactose, 0.5 gm NH4H2PO4, 7 g Na2HPO4, 4 gm KH2PO4, 1 gm CaCl2, 1 gm MgSO4, 1 g peptone and 20 ml trace elements (250 mg FeSO4.7H2O, 80 mg MnSO4.H2O, 70 mg ZnSO4.7H2O, 85 mg CoCl2). pH 5.0. | Seiboth10 et al., (2005).
|
76 |
| VI. | 1 gm lactose, 0.3 gm of malt extract, 0.5 gm peptone and 0.3 gm of yeast extract. pH 6.0. | Robert.Dickson11* et al., 1978 | 88 |
| VII. | 2 gm Lactose, 0.4 gm Peptone,0.4 gm Yeast extract, 0.2 gm KH2 PO4,0.8 gm Na2HPO4 and 0.025 gm MgSO4 pH 6.5. | Zoltan Nagy12 et al., 2001. | 112 |
The typical fermentation course of the isolate MF S4-11 is depicted in Fig 1.1
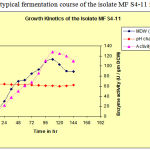 |
Figure 1: The typical fermentation course of the isolate MF S4-11. |
Results and Discussions
The marine strains may have better potential than their terrestrial counterparts. This is due to essential nutrients provided by marine biotopes for nurturing the micro organisms, and to extreme environmental niches. The marine fungi and their by-products may have potential values in food processing, fermentation, pharmaceutical and biopolymer industries. Marine samples were collected from Bay of Bengal near Vishakhapatnam and the samples were screened for marine fungi which resulted, 72 isolates. The fungal isolates were tested for the presence of β-galactosidase activity. Among 72 isolates, 22 isolates exhibited positive response for β-galactosidase activity (both intracellular and extracellular enzyme), of which 5 isolates were found to be potent enzyme producers. As all the isolates are producing more quantity of intracellular enzyme, subsequent studies were devoted to extract endo-enzyme. These isolates were further tested for their β-galactosidase production capabilities by submerged fermentation. The strain MF S4-11 was selected fro the production of β-galactosidase based on the hydrolysis of ONPG. The fermentation studies indicated that the maximum enzyme production at 110 hours of fermentation (Fig 1.1).
Conclusion
The results of this study showed that ocean is a great source of microorganisms, with the possibility of screening of microbes for all kinds of biotechnological uses. The screening and isolation procedures for β-galactosidase from marine source resulted, five potential fungal strains with both intra and extra cellular activity. More than 72 fungal strains were screened for the presence of β-galactosidase. Out of them the isolate MF S2-11 was chosen for further isolation and purification of the enzyme β-galactosidase.
References
- Kathiresan, K., and Thiruneelakandan, G., (2008). Prospects of lactic acid bacteria of marine origin. Indian J. Biotechnol, 7: 178-182.
- Espejo, R.T., and Canelo, E.S., (1968). Properties and characterization of the host bacterium of bacteriophage PM2. J. Bacteriol, 95: 1887-1891.
- Suarez, F.L., Saviano, D.A., and Levitt, M.D., (1995). A comparison of symptoms after the consumption of milk or lactose- hydrolyzed milk by people with self-reported severe lactose intolerance. New England, J. Med, 333: 1-4.
- Rastall, R.A., and Bucke, C., (1992). Enzymatic synthesis of oligo saccharides. Biotechnol. Gen Eng Rev, 10: 253-281.
- Crittenden, R.G., and Playne, M.J., (1996). Production, properties and applications of food grade oligosaccharides. Trends Food Sci Technol, 7: 353-361.
- Francisco, J.Castillo., Harvey, W.Blanch., and Charles, R.Wilke., (1984). Lactase production in continuous culture by Trichoderma reesei RUT-C30. Biotechnol Letters, 6 (9): 593-596.
- Lutz, Fischer., Christian, Scheckermann., and Fritz, Wagner., (1995). Purification and Characterization of a Thermotolerant β-galactosidase from Thermomyces lanuginosus. Applied & Environ Microbiol, 61(4): 1497-1501.
- Phimchanok, Nakkharat., and Dietmar, Haltrich., (2005). Purification and characterisation of an intracellular enzyme with β-glucosidase and β-galactosidase activity from the thermophilic fungus Talaromyces thermophilus CBS 236.58. J. Biotechnol, 123: 304-313.
- Onishi, N., and Tanaka, T., (1997). Purification and characterization of Galacto oligosaccharide producing β-galactosidase form Sirobasidium magnum. Lett. Appl. Microbiol, 24: 82-86.
- Bernhard, Seiboth., Lukas, Hartl., Noora, Salovuori., Karin, Lanthaler., Geoff, D. Robson., Jari, Vehmaanpera., Merja, E. Penttila., and Christian, P. Kubicek., (2005). Role of the bgal-encoded extracellular b-galactosidase of Hypocrea jecorina in cellulase induction by lactose. Appl Environ Microbiol, 71: 851-857.
- Dickson, R.C., Dickson, L.R., and Markin, J.S., (1979). Purification and Properties of an Inducible β-galactosidase isolated from the yeast Kluyveromyces lactis. J. Bacteriol, 137(1): 51-61.
- Zoltan, Nagy., Tunde, Kiss., Attila, Szentirmai., and Sandor, Biro., (2001). β-Galactosidase of Penicillium chrysogenum: Production, Purification, and Characterization of the Enzyme. Protein Expression and Purification, 21: 24-29.







