Mohammad Tawkir1*, S. A. Iqbal2 and S. B. Kapoor2
¹Department of Chemistry, Arts Commerce and Science College Tukum, Chandrapur India.
²Department of Chemistry, Saifia College of Science and Education, Bhopal - 462 001 India.
Abstract
Present Communication deals with the preliminary study fo the comparison of hypoglycemic activity of two mixed sulphonyl urea. i.e. Glibenclamide (5-chloro-N-(4- N-cyclohexyl carbonyl) sulfamoyl] phenethy] 2-methoxybenzamide (L1) adn Glimeperide (3-ethyl-4-methyl-N-(4-[N-(1r,4r)-4-methylcyclohexl carbamoyl) sulfamoyl] phenethyl)-2-oxo-2, 5-dihydro-1-H-pyrrole-1-Carboxamide (L2) in equal proportion with zinc - complex of these mixed drugs result reveals that the ternary (L1ML2) complex of these drugs with zinc gives surprising results.
Keywords
Pharmacological study; Mixed ligands; metal complexes
Download this article as:| Copy the following to cite this article: Tawkir M*, Iqbal S. A, Kapoor S. B. Pharmacological Study of the Glibenclamide and Glimeperide Mixed Ligands and their Zinc Complexes. Biomed Pharmacol J 2010;3(1) |
| Copy the following to cite this URL: Tawkir M*, Iqbal S. A, Kapoor S. B. Pharmacological Study of the Glibenclamide and Glimeperide Mixed Ligands and their Zinc Complexes. Biomed Pharmacol J 2010;3(1). Available from: http://biomedpharmajournal.org/?p=1365 |
Introduction
In recent times zinc – insulin has been successively used as an antidabetic agent To avoid daily pricks of insulin injections. the use of oral antidabetics have increased in recent years.
It is well established fact that a compound or a complex which is to be recommended as a drug of utility must be capable of easy absorption and excretion. it is also essential that neither the substance. it self nor the metallic products there should exercise toxicity or any adverse side effect to the patient.
For this purpose it becomes imperative that following facts must be ascertained for any substance of potential to be used as a drug.
Must not product toxic effects.
Particles size must be of the order of about 5-8 microns for easy absorption.
LD50 causing 50% mortality.
Bacteriostatic tests, where necessary, such tests should be carried out on animals such as rabbit, rat and dogs, when a substance has given satisfactory results for the above tests then only it may be tried on monkeys and men.
However the oral hypoglycemic activity of Chloropropamide.tolbutamide (mixed ligands) and their copper complexes observed by lqbal et-al1 and oral hypoglycemic activity of sulphonamide isopropyl thiadiazoleobseved by janbon et-al2 was followed by hypoglycemic activity of an antibacterial compound carbutamdie by frank and fuchas3. There after Glibenclamide (1) and Glimeperide (ii) being less toxic than other sulphonylureas and were previously well recognized.
It is necessary to find out toxicity. LD50 and animal tests on the metal complex of oral antidiabetic456Iqbal and Co-workers7.8.9 have carried out preliminary pharmacological work for asessing the hypoglycemic activity of Glibenclamide and Glimeperide and compared it with its zinc complex. the results were satisfactory, the hypoglycemic activity was carried out on young dog of approximate 3.5 to 4.5 kg body weight.
Material and Methods
In present work a similar attempt have been taken by way of comparing the result of Hypoglycemic activity of mixed drugs i.e. Glibenclamide and Glimeperide compared to their zinc complex i.e. 1:1:1 (L1ML2)
Glibenclamide – Zn-Glimeperide complex synthesized and its structure was confirmed by analysis and I.R. spectroscopy.
Young dogs approximate body weight 3-4 kg were kept in laboratory conditions for three days. during this period milk and bread at 10.00 am. was given as diet and from the fourth day blood sugar was estimated at 11.00am. which indicated as a first day of the experiment.
Glibenclamide and Glimeperide 50mg (each 25mg) were given orally with small piece of chicken to the young dogs at 10.00 am. These after blood sugar was estimated at 12 pm. and 1.00 pm. The dog was kept on normal diet i.e. bread and milk for three days.
Allowing the blood sugar returning to normal after which 50mg of Glibenclamide – Zn – Glimeperide complex was given orally at 10.05 am. and the blood sugar level (BSL) estimated by ortho – toluidine method (Mono step)10.11.12 calorimetrically and the result are recorded in the table 1 and 2.
Above values were obtained after giving the Dog’s normal diet+GLB/GLP at 9.15 a.m. The dogs were kept on normal diet without drugs for three days.
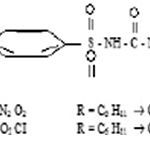 |
Figure 1 |
Colorimetric estimation of % of blood sugar level by Glucose, GOD-POD method (Glucose oxidase – peroxidase (GOD – POD) method).
Principle
D-Glucose+O2 D-Gluconic acid +H2O2 H2O2+4-AAP+P-Hydroxy benzoic acid Sodium + H3O+ Quinone imine + 5H2O2+
Glucose in presence of GOD oxidised to form D-Gluconic acid and H2O2. Thus from react with 4-aminoantipyrine to form red quinoneimine.Quinoneimine thus forms is pink color complex the intensity of which indicates the amount of sugar present in given specimen.
Requriement
Enyzme powder buffer solution (diluent) glucose standard (100mg%)
Preparation of Glucose working solutions
Mix 1 vails of reagent to 1 in a bottle (50ml) of the reagent to diluent utility of solution in 45 days from the date of preparation.
Test procedure
Take 3 clean and dry test tube. test tube label as blank (B), Standard (S) Test (T) Blank solution is any for the selting of photo colorimeter.
Mix and incubate at 370°C for 10 min. or keep at room temperature for 20 min. Take optical density (OD) of standard and test. Using green filter against blank to set zero and absorbance of reagent blank at 270 nm.
 |
Figure 2 |
| Working | 1 ml. | 1ml. | 1ml. |
| Solutions | |||
| 10µl | 10µl | ||
| Glucose | Blood | ||
| standard | serum | ||
Calculations were made using following formula
Results and Dicussion
Before the first day of taking blood sample for glucose estimation Dog’s were kept on normal diet (milk and bread)for three days. Table 2 indicates that the dogs were acclimatized for laboratory conditions with in three days and these after the blood sugar level (BSL) was daily estimated at 9.00a.m. before meals. the average values of all the five days were carried out i.e. 104, 97,4,84,6,4,87.6 mg/dl blood for five days On the day 6th GLB+GLP 50 mg was given at 9.00 a.m. and blood sugar (BSL) level were estimated at 11.10 a.m. and 1.00 pm and the results were recorded in table 2.
Table 2: Comparison of hypoglycemic activity of Glibenclamide and Glimeperide with their zinc complex
| Days of taking | Drug dose | Diet | Time | Glucose estimated in mg/dl | ||
| blood sample | and time | 9.00 a.m | 11.00 a.m | 1.00 p.m. | ||
| Day Ist | Nill | Milk & Bread | 11.00 am | 104 | 104 | 103 |
| Day 2nd | Nill | Milk & Bread | 11.00 am | 97.4 | 97.5 | 97 |
| Day3rd | Nill | Milk & Bread | 11.00 am | 84.6 | 85.6 | 85.4 |
| Day 4th | Nill | Milk & Bread | 11.00 am | 82.6 | 82.8 | 82.6 |
| Day 5th | Nill | Milk & Bread | 11.00 am | 87.6 | 87.8 | 87.4 |
| Day 6th | GLB/GLP | Fasting | 11.00 am | 84.4 | 53.4* | 44.7* |
The similar process was applied to young male dogs for estimating blood sugar level (BSL) by way of giving orally GLB-Zn-GLP complex in ratio to taking 50 mg/kg body weight. Before performing the experiment of oral administration of mixed ligand complex. The animals were kept for normal diet for three days in order to maintain their normal blood sugar level (BSL).
Above values were obtained after giving the Dog’s normal diet+GLB/GLP at 9.15 a.m. The dogs were kept on normal diet without drugs for three days
Days 10th GLB-Zn-GLB complex 50mg fasting 11.00 a.m. 86.2 51.4 42.8
Acknowldegements
The author are thankful to Dr. S.A. iqbal Head of department of chemistry Saifia College Bhopal, Thankful to Dr. M.M. Wankhede, Principal A.C.S. College Chandrapur for constant encouragement and providing necessary facilities.
References
- Arshi siddique, S.A.Iqbal, Suman Malik and Meena Iqbal Indian J. Applied & Pure Bio. 20(2): 227-230 (2005).
- Janban, M.I. Chaptal, A. Vedal and J.Schap, Montpell – Med, 441: 21-22 (1962).
- Frank H. and J.Fuchs, Devt Med. Wochschr80: 1149 (1995).
- Base B.C., Text book of pharmacology Scientific Book agency. Calcutta 96 (1995).
- Burry J.H. Goodwin, Biological standardisation oxford univ. press london PII (1952).
- The Indian pharmacopea, Govt. of Indian 1nd Ed. P409, 1017 1032 (1956).
- Iqbal, S.A., N.U. Siddique and S.M. Khan, Indian J.Applied and Pure Biol. 2(2): 65-67 (1987).
- Iqbal S.A. I. Husain, M.A. khan, N.A.Khan and N.U.Siddique, Orient J.Chem. 3(2): 199-202 (1987).
- Qureshi R. and Iqbal S.A., Indian J. Applied and Pure Biol. 2(2) 65-67 (1987).
- Trinder P. Glucose oxidase method Arm. ClinBiochem. 6: 24 (1969).
- Thomas L. Clinical laboratory Diagnostics.1st Ed. Frankfurt. : Th-Books veriagsgesellschaft, 374-7 (1998).
- Burtis CA. Ashwood ER, editors, Tietz Text book of clinical chemistry 3rd Ed. philadelphia : W.B. Saunders company, 1838 (1999).
- Tietz NW, Ed. clinical Guide to laboratory Tests, 3rd Ed. Philadelphia, Pa: W.B. Saunders company, 268-273 (1995).







