Alok. Pareek*, P. E. Joseph and Daya S. Seth
School of Chemical Sciences, Department of Chemistry, ST. John’s College, Agra - 282002 India.
Abstract
A simple synthesis of various substituted coumarin and Schiff base was prepared by the condensation reactions of (R) phenyl malon anilic acid (1a, 2a) with different substituted salicylald ehydes in the presence of pyridine as a catalyst at maintained temperature. Substituted benzo-coumarin and benzo-Schiff base has been synthesized by the condensation reactions of (R) malon anilic acid (1a, 2a) with 2-hydroxy-1-Naphthaldehyde in the presence of pyridine. The constitution of the newly synthesized compounds are characterized by their spectral studies like IR, 1H NMR, elemental analysis, physical properties. Further they were assayed for their antibacterial activity against one Gram + ve S.aureus and one Gram - ve E.coli micro-organism.
Keywords
Substituted Coumarin and Schiff base; Benzo-coumarin and Benzo-Schiff base; Pyridine; Malonic acid
Download this article as:| Copy the following to cite this article: Pareek A, Joseph P. E, Daya S. SethNovel Synthesis, Characterization of some New Substituted Coumarin and Schiff Base, Substituted benzo-coumarin and benzo-Schiff Base and Study of their Antibacterial Activities. Biomed Pharmacol J 2010;3(1) |
| Copy the following to cite this URL: Pareek A, Joseph P. E, Daya S. SethNovel Synthesis, Characterization of some New Substituted Coumarin and Schiff Base, Substituted benzo-coumarin and benzo-Schiff Base and Study of their Antibacterial Activities. Biomed Pharmacol J 2010;3(1). Available from: http://biomedpharmajournal.org/?p=1270 |
Introduction
Coumarin is a simple oxygen containing heterocyclic compound, present in Melilot and Tonca bean. It is the Odoriferous Principle Wood-ruffs which led to its wide spread used as perfuming in chemical industry. Several coumarin derivatives have been found to possess antibiotics1, antiinflammatory2, antibacterial3, anti-fungal4 activities. Several anti-bacterial drugs are modulate on coumarin’s structure as Novobiocin coumeromycin. Substituted coumarin derivatives have also been reported to possess antiallergic5, anticoagulant6, antidiabetic7 activities. On the other hand the schiff’s base has long shown pharmacological interest as antitubercular8, anti- cancer9, antitumor10, fungicidal11, agro-chemical12 activities.
Some of the substituted benzo-coumarin & schiff’s base derivatives have been possesses antibacterial13, fungicidal14 activity.
By various workers several substituted coumarins & schiff’s base and benzo-coumarin & benzo-schiff’s base have been synthesized in our laboratory15-17. In the present study we have synthesized a novel series of some new substituted coumarin & schiff’s base by the condensation of 2-methoxy-5-methyl, 3 -chloro- 4-methoxy malon anilic acid with different substitut-ed salicylaldehydes in the presence of pyridine and the benzo-coumarin & benzo-schiff’s base was prepared by the condensation reactions of 2-methoxy-5-methyl, 3-chloro-4-methoxy malon ani-lic acid with 2-Hydroxy-1-Naphthaldehyde in the presence of a catalyst as pyridine.
Experimental
Material and Methods
All the chemical used in the synthesis were obtained from Sigma-Aldrich Company. All the melting points of newly synthesized compounds were determined in open capillary tubes and were uncorrected. All the novel compounds were recrystallized by absolute ethanol 99%. The purity of the synthesized compounds was checked by TLC. The Infrared spectra (cm-1) were recorded on Perkin-Elmer Spectrum RX-1 FT-IR spectroph-otometer at ST. John’s College, Agra. 1H NMR spectra was measured on Advance Bruker DRX-300 using hexadeuterio dimethyl sulfoxide (DMSO d6 ), chemical shift are given in(ppm) and protons signals are indicated as: s = singlet, d = doublet, t = triplet, m = multiplet. Analytical and physical analysis of synthesized compounds were listed in the Table-1.
Synthesis of N(R)-phenyl malon anilic acid (1a, 2a),N : NI – di(2-methoxy-5-methyl) phenyl malonamide (3a), ethyl N (2-methoxy-5-methyl) phenyl malonamate (3b), N(2-methoxy-5-meth-yl) phenyl malonamic acid amide (3c).
To the primary amine (1a,2a;0.025 mole), diethyl malonate (0.05 mole) was added in the presence of a catalyst (DMF), refluxed for 45 – 60 minutes, cooling, filtered. Take solid part of (1a) and recrystallized by absolute ethanol, on analysis it was identified as N:NI-di(2-methoxy-5-methyl) phenyl malonamide (3a), the main filtrate of (1a) was collected in china-dish and concentrated it on boiling water-bath, the coloured mass of the compound was treated with 20 ml portion of petroleum ether (100-120OC), then recrystallized, it was found to be ethyl N-(2-methoxy-5-methyl) phenyl malonamate (3b), ethyl N-(2-methoxy-5-methyl) phenyl malonamate (0.01mole;3b) was dissolved in 15 ml of ethanol in r.b.flask was added with liquor NH3 (15 ml), the flask was tightly corcked, vigorously shaken, cooling, the crystalline solid was obtained, filtered, purified by absolute ethanol, it was found to be N(2-methoxy-5-methyl) phenyl malonamic acid amide (3c). In the filtrates of main product add ethyl alcohol with the solution of Na2CO3, take hydrolysis of the main mixture for 45 minutes, filtered, to the filtrate Con. HCI was added slowly. Thus the solid was separated, filtered, washed with distilled water, recrystallized, was identified to be N(R) phenyl malon anilic acid (1a, 2a).
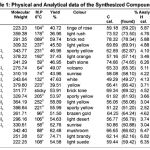 |
Table 1 |
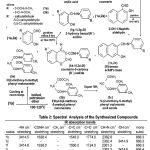 |
Table 2 |
Synthesis of coumarin-3-carboxy-(RI) anilide (1d-1f, 2d-2f)and 2-hydroxy benzal (RI) aniline (1g-1i, 2g-2i)
A mixture of (1a, 2a ; 0.001 mole) and substituted salicylaldehydes (0.001 mole) with a trace of pyridine, then the reaction mixture was heated for 4-hours in an oil-bath at 104-110OC, after cooling it was digested with the solution of NaHCO3, washed with water, the schiff’s base was removed by the extraction with hot ethanol (15ml),take the ethanolic extract and concentrate it, cooling, gave 2-hydroxy-benzal (R) aniline. The residue was recrystallized from hot ethanol as identified to be coumarin 3-carboxy-(R) anilide.
Synthesis of substituted benzo – coumarin (1b, 2b) and benzo – schiff’s base (1c, 2c)
A mixture of ( 1a,2a;0.01 mole) and 2-OH-1-Naphthaldehyde(0.01 mole) with few drops of pyridine , heated for 4-hours in an oil-bath, the mixture was first melted and then soon set to a solid, cooling, and then digested with Sodium bicarbonate saturated solution(20 ml). The alkali extract then decanted and residue was washed with water. The residue was boiled with hot ethanol and filtered, the ethanolic extract was concentrated, after cooling, gave substituted benzo-schiff’s base.
The residue left after boiling with absolute ethanol was recrystallized as yellow crystals of substituted benzo-coumarin.
Antibacterial activity
The antibacterial activity of synthesized compounds was investigated by employing the filter paper disc method18-19 was followed by using special Hi-Media Sterile disc code SD-067, representative organisms selected for evaluation of antibacterial activity were S.aureus and E.coli. The compounds was evaluated at 25 µg ml-1 concentration. The compounds were tested as a solution or suspension in DMF. An important and useful control drug streptomycin also tested under similar conditions, with view to compare the results.
Results and Discussion
The IR Spectra (Kbr-disc) of the newly synthesized compounds have been recorded in the frequency region 4000-500 cm-1 and the 1H NMR spectral data are recorded in the Table-2.
The Infrared spectra of the compound showed absorption bands at 3414.5 cm-1(broad peak –NH),1638.4 cm-1 (CONH str.), 1543.0 cm-1 (C=C str.), 1774.0 cm-1(lactone C=O), 2362.0(CH=N str.), 668.3 (mono substitution str.) and 2362.4 cm-1(CH=N str.), 3414.9(-OH group str.), 668.3 (linkage). These results of compounds 1b and 1c indicates the absorption spectrum was in agreement with the assigned structure.
The compound (1f,1i) showed absorption bands at 3414.8 cm-1 (-NH str.),1638.0 cm-1 (CONH str.), 1560.0 cm-1 (C=C str.), 1724.0 cm-1(lacto-ne C=O), 2361.0(CH=N str.), 668.3 (mono substitution str.) and 2362.3 cm-1(CH=N str.), 3414.6(-OH group str.), 668.4 (linkage).The infrared spectral results indicates the absorption spectrum was in agreement with assigned structure of compound (1f, 1i) and other compounds (1d-1e, 1g,1h).
The Infrared spectra of the compound (2b) showed absorption bands at3414.8 cm-1(-NH str.), 1638.1 cm-1 (CONH str.), 1560.7 cm-1 (C=C str.),1774.0 cm-1(lactone C=O), 2362.7(CH=N str.), 668.3 (mono substitution str.). Thus these observati-ons lent support to the assigned structure. The compound (2f,2i) showed absorption bands at 3414.2 cm-1 (-NH str.),1638.2 cm-1 (CONH str.), 1501.4 cm-1 (C=C str.), 1708.0 cm-1(lactone C=O), 2362.1(CH=N str.), 667.3 (mono substitution str.) and 2361.4 cm-1(CH=N str.), 3414.2(-OH group str.), 668.4 (linkage). The infrared spectral results indicates the absorption spectrum was in agreement with assigned structure of compound (2f, 2i) and other compounds (2d-2e, 2g,2h).
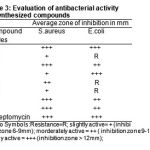 |
Table 3: Evaluation of antibacterial activity of synthesized compounds |
The 1H NMR spectra showed singlet at δ 2.219 (COOH),7.878(Ar-H),9.469(-CONH),doublet at δ 3.469(-CH2),multiplet at δ 6.935(ring),10.160(CONH), confirming the structure of compound (1a). The results indicates that the compounds showed moderate to highly activity against these bacterial strains, all results are listed in the Table-3.
Acknowledgements
The author is thankful to Head Central Drug Research Institute (CDRI), Lucknow for spectral data (1H NMR) and Head, Department of Botany, R.B.S. College, Agra for biological screening.
References
- H.Wallick, D.A.Harris, M.A.Reagan, M.Rugir & H.Wooduff, Antibiot. Ann., 909(1956)
- Ghata, Manjunath, D.Manohar, V.Kulkarni, R.Shobha, S.Y.Katrimani, Euro.J.Med.Chem.,38(3) ;297(2003)
- Raviraj Kusanur, Manjunath Chata, Kulkarni, Manohar, Ind. J. Heterocycl. Chem., 13(3);201(2004)
- K.Ranjander, D.Karunkar, M.Shrinivas,Indian J.Chem., 43B; 643(2004)
- K.Ukawa, Y.Ishiguro, A.Wada, Nohara, Heter ocycles, 24, 1931(1986)
- H.Brauninger, R.Plagemann, H.D.Schalicke, K.Peseke, Naturwissen Schaftliche Reiche, 35, 34-39(1986)
- D.Heber, Arch. Pharm., 320, 402-406(1987)
- Marchant, R.Jaysukhlal and D.S.Chothia, J. Med.Chem., 13(2), 335(1970)
- M.S.Shingare and D.B.Ingle., J.Indian Chem. Soc., 53, 1036(1976)
- Deliwala and Chimanlal, J.Med.Chem., 14(5),450(1971)
- P.R.Panditrao, S.D.Deval, S.M.Gupta, S.D.S amnt and L.D.Deodhar., Indian J.Chem.,20(B) , 929(1981)
- DincerSebla, Indian J. Chem., 33(B), 1335 (1999)
- R.P.Pawar, N.M.Andurkar, Y.B.Vibhute, Orient.J.Chem., Vol.15(1), 157-160(1999)
- V.P.Singh, R.V,Singh and J.P.Tandon, Main group, Med.Chem.,13(3),135-48(1990)
- R.K.Jain, Ph.D.Thesis,Agra Univ., Agra(1978)
- A.K.Mittal,Ph.D.Thesis,Agra Univ.,Agra(1981)
- D.S.Seth,Ph.D.Thesis,Agra Univ.,Agra(1972)
- R.Cruickshank, J.P.Duguid, B.P.Marion and RHA, Medicinal Microbiology,12th Edn.,2,196-202(1975)
- L.J.Bradshaw Ed., A text book of Microbiology, W.P.Sounders Co., Philadelphia, New York (1979)







