Rajasekhar Pinnamaneni and Sankar Jagarlamudi
Centre for Biotechnology, Acharya Nagarjuna University, Nagarjuna Nagar - 522 510 India.
Abstract
Genetic variants of the endothelial nitric oxide synthase (eNOS) gene for 27bp-VNTR have been examined for their association with type 2 diabetes (T2DM)-related traits in various populations of Andhra Pradesh, India. The carriers of the rare allele (27bp-VNTR-4a) were found to have decreased HDL-C and increased DBP levels. Genetic polymorphisms examined at the eNOS locus, 27bp-VNTR appears to be a minor contributor to the variation in T2DM-related traits in populations of Andhra Pradesh, India.
Keywords
eNOS gene; genetic polymorphisms; type 2 diabetes
Download this article as:| Copy the following to cite this article: Pinnamaneni R, Jagarlamudi S. Genetic Variants in Diabetes Mellitus Patients by Amplifying the Intron of eNOS Gene in the Population of Andhra Pradesh, India. Biomed Pharmacol J 2010;3(1) |
| Copy the following to cite this URL: Pinnamaneni R, Jagarlamudi S. Genetic Variants in Diabetes Mellitus Patients by Amplifying the Intron of eNOS Gene in the Population of Andhra Pradesh, India. Biomed Pharmacol J 2010;3(1). Available from: http://biomedpharmajournal.org/?p=1127 |
Introduction
Nitric oxide (NO) plays a fundamental role in the regulation of endothelial function and vascular tone in many organs, including the kidney. NO inhibits platelet aggregation, leukocyte adhesion to vascular endothelium and oxidation of low-density lipoprotein (LDL) [1]. Endothelium-derived NO is synthesised from L-arginine by NO synthase encoded by the endothelial NO synthase (eNOS or NOS3) gene, mapped to chromosome 7q36. Upon release, NO diffuses rapidly through the cell membrane and relaxes neighbouring vascular smooth cells through the production of cyclic guanine 3’5’-monophosphate (cGMP). cGMP then activates the protein kinase G family, leading to a cascade of responses at the levels of transcription and translation. The increase in cGMP levels forms the basis for regulation of several physiological functions, including relaxation of vascular smooth muscle cells. Impairment of NO production causes endothelial dysfunction, which contributes to the development of insulin resistance, type 2 diabetes mellitus (T2DM), chronic renal failure, and cardiovascular complications including hypertension and hypercholesterolaemia [2]. Due to the importance of eNOS in the generation of NO that regulates endothelium dependent vasodilation in many organs; several epidemiological studies are in the process of evaluating whether genetic polymorphisms in the eNOS gene, and alteration in eNOS activity and NO availability, are associated with T2DM, cardiovascular and renal diseases.
Over the last few years, several polymorphisms of the eNOS gene have been identified, and their association with various diseases has been explored. In particular, a singlenucleotide polymorphism (SNP) in the promoter region (T-786C), a G to T substitution at nucleotide 894 in exon 7 of eNOS, which leads to an amino acid change from Glu to Asp at codon 298 (Glu298Asp), and a 27 bp variable number of tandem repeats (27bp-VNTR) polymorphism in intron 4 have received most of the attention, because of their functional relevance to eNOS activity. The T-786C promoter polymorphism reduces the promoter activity and thus affects eNOS protein expression and eNOS activity [3]. The Glu298Asp polymorphism causes a structural change of the eNOS protein and reduces eNOS activity. In the 27-bp repeat of intron 4, two alleles have been identified, the larger of which, eNOS-4b, has five tandem 27-bp repeats (GAAGTCTAGACCTGCTGC(A/G) GGGGTGAG) and the smaller, eNOS-4a, has four repeats. The 27bp-VNTR reduces the plasma concentration of nitric oxide [4]. However, genetic studies examining the association of T-786C, Glu298Asp and 27bp-VNTR polymorphisms with cardiovascular and renal diseases have yielded conflicting conclusions [5].
The aim of the present study was therefore to investigate the polymorphism in intron of eNOS3 discussed above associated with type 2 diabetes, a study in the population of Andhra Pradesh, India.
Materials and Methods
Study subjects and phenotypic data
Probands were low-income population of Andhra Pradesh, India with T2DM, and all first, second and third degree relatives of probands were invited to participate in the study. A variety of metabolic, haemodynamic, anthropometric and demographic variables were collected from about 500 individuals drawn from 40 large native families. Studies were conducted by drawing the blood samples using standard procedures. Blood samples were obtained after a 12-hour fast for assessment of various phenotypes including glucose, total cholesterol, triglycerides (TG) and high-density lipoprotein cholesterol (HDL-C), and they were collected again two hours after a standardised oral glucose load to measure plasma glucose. Measurement of all these phenotypes has been described elsewhere [6]. Diabetes status was defined by the 1999 criteria of the World Health Organization (i.e. fasting glucose levels > 126 mg/dL and/or two-hour glucose levels > 200 mg/dL). Participants who did not meet these criteria but who reported that they were being treated with either oral antidiabetic agents or insulin and who gave a history of diabetes were also considered to have T2DM. Urine albumin excretion was expressed as the albumin to creatinine ratio (ACR) [7] and analysed as a continuous variable. The quantitative trait values of triglycerides (ln TGL) and ACR (ln ACR) were log-transformed for the association analyses since their raw data were not normally distributed. All the subjects gave their informed consent.
Statistical genetic analysis
The genotypic data were checked for Mendelian pedigree inconsistencies using the programs INFER and GENTEST (PEDSYS programs) [8]. Allele frequencies were estimated, and all polymorphisms were tested for Hardy–Weinberg equilibrium. We performed association analysis in our family data using the measured genotype approach (MGA) within the variance components (VC) analytical framework [9]. The VC based approach accounts for the non-independence among family members. In this approach, VCs are modelled as random effects (e.g. additive genetic effects and random environmental effects), whereas the effects of measured covariates such as age and sex are modelled as fixed effects on the trait mean. The marker genotypes were incorporated in the mean effects model as a measured covariate, assuming additivity of allelic effects [9, 10, 11]. The effect of this measured genotype (association parameter) together with other covariate effects (such as age and sex) and VCs were estimated by maximum likelihood techniques. The hypothesis of no association is tested by comparing the likelihood of a model in which the effect of the measured genotype is estimated with a model where the effect of the measured genotype is fixed at zero. Twice the difference in the log-likelihoods of these models yields a test statistic that is asymptotically distributed, approximating a χ2 distribution with one degree of freedom. A p value < 0.05 is considered significant. Prior to performing MGA, the quantitative transmission disequilibrium test (QTDT) [12] was used to examine hidden population stratification. All statistical techniques described above were implemented in the computer program SOLAR [http:// www.sfbr.org/solar/] [10].
Isolation and amplification of DNA from blood of Diabetic patients
| Primers | Sequence |
| Forward | 5I– AGGCCCTATGGTAGTGCCTTT-3I |
| Reverse | 5I– TCTCTTAGTGCTGTGGTCAC-3I |
DNA was prepared by standard procedures from leukocytes [13]. The 27bp-VNTR polymorphism in intron 4 was amplified in a Master cycler gradient (Eppendorf, Germany) programmed at 5 minutes. In Polymerase Chain Reaction, the specific primers (Forward and Reverse) were used to amplify the genomic sequence of the in intron 4. The master mix containing 10X Taq buffer, 10 mM dNTPs, 25 mM of MgCl2, 1 U of Taq DNA polymerase,1.5 μl of forward primer, 1.5 μl of Reverse primer, 100 ng of genomic DNA and PCR grade molecular water were made to the final volume 20 μl was used. Taq DNA polymerase initiates the replication of DNA fragments by using nucleotide base from dNTP mixture (A, T, G, and C). Linear amplification was performed at 95°C for 5 minutes, followed by 40 cycles of 30 s of denaturation at 94°C, 30 s of annealing at 60°C, and 3 minutes of polymerization at 72°C and then 72°C for 3 minutes. Exponential amplification was performed at 95°C for 5 minutes, followed by 35 cycles of 30 s of denaturation at 94°C, 30 s of annealing at 60°C, and 2 minutes of polymerization at 72°C and then 72°C for 10 minutes. The PCR products were analyzed by Agarose gel Electrophoresis on a 1.6 percent agarose gel, visualized under UV light, photographed and documented with an Alpha Imager (Alpha Innotech, California, USA).
Agarose gel electrophoresis
3% agarose (w/v) was weighed and melted in 1X TBE buffer (0.9M Tris-borate, 0.002 M EDTA, pH 8.2). Then, 1-2 µl ethidium bromide was added from the stock (10 mg/ml). After cooling, the mixture was poured into a casting tray with an appropriate comb. The comb was removed after solidification and the gel was placed in an electrophoresis chamber containing 1X TBE buffer. The products were mixed with 6X loading buffer (0.25% bromophenol blue, 0.25% xylene cyanol FF, 30% glycerol in water) at 5:1 ratios and loaded into the well. Electrophoresis was carried out at 60V [14]. The wildtype allele (five copies of 27 bp repeats – b allele) generated a 420 bp band and the mutant allele (four copies of 27 bp repeats – a allele) generated a 393 bp band.
Results
The clinical characteristics of the subjects who provided genotypic and phenotypic data for this study were shown in table.1. Of the genotyped individuals, the mean age of the study subjects was 43 years, and 55% of them were females. Of the total sample, 27% of the subjects had T2DM.
Table 1: Clinical characteristics of the genotyped SAFDGS participants used in the present study
| Variables | Mean + SD or % |
| Females | 55 |
| Type 2 diabetes | 27 |
| Age (yrs) | 45.5±10.2 |
| Systolic blood pressure (mmHg) | 126.4±15.6 |
| Diastolic blood pressure (mmHg) | 70.3±9.6 |
| Body mass index (kg/m2) | 30.9±5.0 |
| Total cholesterol (mg/dL) | 193.0±35.4 |
| High-density lipoprotein cholesterol (mg/dL) | 46.0±11.5 |
| Triglycerides (log-transformed) | 4.5± 0.5 |
| Albumin to creatinine ratio (log-transformed) | 2.5±0.7 |
DNA extraction, Purification and Quantification
The DNA pellet from the blood samples of Diabetic and normal patients obtained after washing with 70% ethanol appeared like white, thick thread like mass. This DNA obtained was further quantified by spectrophotometry and agarose gel electrophoresis. It was observed that blood DNA fragments were observed to emit orange fluoroscence under UV lamp. The A260/A280 ratio for blood DNA was found to be 1.9 spectrophotometrically. This study revealed the method adopted for extraction; purification and quantification of DNA were found to be suitable for molecular studies on Diabetics. The concentration of DNA was adjusted to 0.3 µg/mL with sterile distilled water for carrying out the amplification reactions.
Amplification of intron gene of Diabetic patients by Polymerase Chain Reaction (PCR)
The amplified fragment of DNA analyzed on agarose gel electrophoresis was of good quality. 27bp-VNTR polymorphism was carried out, as described. The amplified fragment molecular mass is around 420-450bp. 27bp-VNTR was consistent with the Hardy-Weinberg equilibrium expectations, and there was no evidence for hidden population stratification in the data as tested by Quantitative trait determinants.
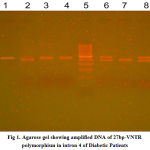 |
Fig.1: Agarose gel showing amplified DNA of 27bp-VNTR polymorphism in intron 4 of Diabetic Patients. Wells 1, 3, 4: 4a/a; Wells 2 and 7: 4b/b; Wells 6 and 8: 4b/a; Well 5: DNA Ladder. |
Genotyping of 27bp-VNTR polymorphisms was carried out, as described earlier. The allele and genotype frequencies are presented in table 2. Genotypic data of 27bp-VNTR were consistent with the Hardy-Weinberg equilibrium expectations, and there was no evidence for hidden population stratification in the data as tested by QTDT.
Table 2. Allele and genotype frequency of the selected variants from the eNOS gene
| Polymorphisms | Major/minor allele (%) | Genotype (%) | ||
| 27bp-VNTR – a (4 repeats)/
b (5 repeats) |
b (90) /
a (10) |
aa (1) | ab (18) | bb (81) |
Association analysis in our family data was performed using the MGA. A number of phenotypes were examined for association–T2DM, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol, HDL-C, ln TGL and ln ACR. After adjusting for the traitspecific covariate effects (table 3), only HDL-C (p=0.04) and DBP (p=0.02) exhibited significant association with 27bp- VNTR polymorphism.
Table 3. Association analysis between NOS3 gene polymorphisms and diabetes-related
| Phenotypes | 27bp-VNTR
p Value |
| Type 2 diabetes | 0.57 |
| Body mass index | 0.79 |
| Total cholesterol | 0.3 |
| High-density lipoprotein cholesterol | 0.04 |
| Triglycerides | 0.52 |
| Systolic blood pressure | 0.42 |
| Diastolic blood pressure | 0.02 |
| Albumin to creatinine ratio | 0.85 |
Table 4 shows the 27bp-VNTR genotype class-specific mean values of HDL-C and DBP levels. As can be seen, the carriers of a allele (four repeats of 27bp-VNTR) were found to have decreased HDL-C and increased DBP levels.
Table 4. Mean values of HDL-C and DBP levels by 27bp-VNTR genotype category
| Phenotypes | Mean + SE by genotypes | ||
| a/a (4 repeats) | a/b | b/b (5 repeats) | |
| HDL-C (mg/dL) | 37.9±2.6 | 40.5±1.5 | 43.1±1.0 |
| DBP (mmHg) | 79.1±1.9 | 77.0±1.1 | 74.9±0.8 |
HDL-C = high-density lipoprotein cholesterol; DBP = diastolic blood pressure
Discussion
Endothelial nitric oxide synthase, a key regulator of vascular nitric oxide production, has been investigated extensively to determine the relevance of DNA variants in the eNOS gene to vascular and renal diseases. The variants in the promoter region intron-4 (27bp-VNTR) have been explored in several epidemiological studies, but the association findings have been inconsistent. Also, the genetic association studies examining these polymorphisms have been conducted. To our knowledge, the genetic epidemiological studies examining these polymorphisms in the population of Andhra Pradesh for their association with cardiovascular and renal disease-related traits are very limited [5]. There is a marked ethnic difference in the distribution of eNOS variants. The studies on the distribution of 27bp-VNTR variants of the eNOS gene in a sample of 305 individuals of different ethnicity (100 Caucasians, 100 African Americans and 105 Asians) revealed that the 786C variant was more common in Caucasians (42%) than in African Americans (18%) or Asians (14%). With respect to 27bp-VNTR polymorphism, the minor allele frequency of a allele (four repeats) was more common in African Americans (27%) than in Caucasians (16%) or Japanese (13%) [15], the frequency of a allele (four repeats) in Mexican Americans (10%) was similar to that reported in a Venezuelan Hispanic population [16]. Such ethnic differences in the occurrence of genetic variants at the eNOS locus may relate to the ethnic-specific predisposition to T2DM and its correlated phenotypes.
In this study, we evaluated the phenotypic association between measures of T2DM and its correlated cardiovascular, renal-related traits and genotypes of 27bp-VNTR polymorphisms of the eNOS gene in the population of Andhra Pradesh at high risk for the development of T2DM and related risk factors. While our study failed to find significant association between the 27bp-VNTR exhibited nominally significant association with HDL-C and DBP measures after adjusting for the trait specific covariate effects. Our results are in agreement with other reports in Asians and Caucasians [1, 17, 18, 19]. The 27bp-VNTR four repeats (a allele) carriers were found to have lower HDL-C values and higher DBP values. Few studies have reported that 27bp-VNTR polymorphisms are in perfect linkage disequilibrium (LD). Such a LD pattern between these two polymorphisms was not seen in our study.
This study suggests that further examination of the association found between 27bp-VNTR and HDL-C or DBP levels is warranted. There are limitations of the findings in this study. First, we only investigated three polymorphisms that have been extensively examined by other studies, instead of examining comprehensive tagging of all common variations within the eNOS gene that could influence phenotypic variation in our population. Second, once the issue of multiple testing is considered, the two nominally significant associations found in this study become insignificant. However, we believe that our findings could contribute to any future meta-analyses validating these polymorphisms.
Conclusion
The study revealed that there was some evidence for a minor contribution of the 27bp-VNTR polymorphism to variation in T2DM related subclinical cardiovascular traits in population of Andhra Pradesh, India.
References
- Casas, J.P., Cavalleri, G.L., Bautista, L.E., Smeeth, L., Humphries, S.E. and Hingorani, A.D. Endothelial nitric oxide synthase gene polymorphisms and cardiovascular disease: a HuGE review. Am J Epidemiol; 164:921-35 (2006).
- Wang, X.L. and Wang, J. Endothelial nitric oxide synthase gene sequence variations and vascular disease. Mol Genet Metab; 70:241-51 (2000).
- Nakayama, M., Yasue, H. and Yoshimura, M. T-786 C mutation in the 5′-flanking region of the endothelial nitric oxide synthase gene is associated with coronary spasm. Circulation; 99:2864-70 (1999).
- Tsukada, T., Yokoyama, K. and Arai, T. Evidence of association of the ecNOS gene polymorphism with plasma NO metabolite levels in humans. Biochem Biophys Res Commun; 245:190-3(1998).
- Guo, X., Cheng, S. and Taylor, K.D. Hypertension genes are genetic markers for insulin sensitivity and resistance. Hypertension;45:799- 803 (2005).
- Puppala, S., Dodd, G.D. and Fowler, S.A. genomewide search finds major susceptibility loci for gallbladder disease on chromosome 1 in Mexican Americans. Am J Hum Genet;78:377-92 (2006).
- Lehman, D.M., Leach, R.J. and Johnson-Pais, T. Evaluation of tight junction protein 1 encoding zona occludens 1 as a candidate gene for albuminuria in a Mexican American population. Exp Clin Endocrinol Diabetes;114:432-7 (2006).
- Dyke, B. PEDSYS: a pedigree data management system. Tech rep 2, Population Genetics Laboratory, Department of Genetics, Southwest Foundation for Biomedical Research, San Antonio (1996).
- Boerwinkle, E., Chakraborty, R. and Sing, C.F. The use of measured genotype information in the analysis of quantitative phenotypes in man. I. Models and analytical methods. Ann Hum enet;50:181-94 (1986)
- Almasy, L. and Blangero, J. Exploring positional candidate genes: linkage conditional on measured genotype. Behav Genet;34:173-7 (2004).
- Richardson, D.K., Schneider, J. and Fourcaudot, M.J. Association between variants in the genes for adiponectin and its receptors with insulin resistance syndrome (IRS)-related phenotypes in Mexican Americans. Diabetologia;49:2317-28 (2006).
- Abecasis GR, Cookson WOC, Cardon LR. Pedigree tests of transmission disequilibrium. Eur J Hum Genet 2000;8:545-51.
- Pociot, F., M¢lvig, J. and Wogensen, L. A tumour necrosis factor beta gene polymorphism in relation to monokine secretion and insulin-dependent diabetes mellitus. J Scand Immunol; 33:37- 49 (1991).
- Sambrook, J., Fritsch, E.F, and Maniatis, T. Molecular cloning: A laboratory manual. Cold Spring Harbor Laboratory, New York. (1989).
- Tanus-Santos, J.E., Desai, M. and Flockhart, D.A. Effects of ethnicity on the distribution of clinically relevant endothelial nitric oxide variants. Pharmacogenetics;11:719-25 (2001).
- Hoffmann, I.S., Tavares-Mordwinkin, R., Castejon, A.M., Alfieri, A.B. and Cubeddu, L.X. Endothelial nitric oxide synthase polymorphism, nitric oxide production, salt sensitivity and cardiovascular risk factors in Hispanics. J Hum Hypertens;19:233-40 (2005).
- Benjafield, A.V. and Morris, B.J. Association analyses of endothelial nitric oxide synthase gene polymorphisms in essential hypertension. Am J Hypertens;13:994-8 (2000)
- Pulkkinen, A., Viitanen, L., Kareinen, A., Lehto, S., Vauhkonen, I. and Laakso, M. Intron 4 polymorphism of the endothelial nitric oxide synthase gene is associated with elevated blood pressure in type 2 diabetic patients with coronary heart disease. J Mol Med;78:372-9 (2000).
- Ohtoshi, K., Yamasaki, Y. and Gorogawa, S. Association of (-)786T-C mutation of endothelial nitric oxide synthase gene with insulin resistance. Diabetologia;45:1594-601 (2002).







