G. Roopa1*, R. Sudeendra Bhat2 and Murthy Sudarshan Dakshina2
1Sarada Vilas College of Pharmacy, Mysore India.
2J. S. S. College of Pharmacy, Mysore India.
Abstract
Herbal drugs like Glycyrrhiza glabra, Terminalia chebula, Terminalia belerica, Emblica officinalis and mineral Turbinella rapa possess anti-phlogistic, astringent and acid neutralization activity that is desirable for the treatment of gastric ulcer. The extracts of the herbs were obtained by cold maceration process and their extracts were formulated into a suspension with the varied concentration of Xanthan gum. The suspensions were then evaluated for the pH, Viscosity, sedimentation volume, redispersibility, antacid and antiulcer activity. All the formulations showed a pH in the basic range about 8.2, acid neutralizing capacity between 2-3mEq/ml, high sedimentation volume and good redispersibility .The formulation containing extracts of the herbs showed significant decrease in the ulcer index as compared to one containing the powders of these drugs .
Keywords
Glycyrrhiza glabra; Turbinella rapa; Antiulcer activity; Antacid activity
Download this article as:| Copy the following to cite this article: Roopa G, Bhat R. S, Dakshina M. S. Formulation and Evaluation of an Antacid and Anti-Ulcer Suspension Containing Herbal Drugs. Biomed Pharmacol J 2010;3(1) |
| Copy the following to cite this URL: Roopa G, Bhat R. S, Dakshina M. S. Formulation and Evaluation of an Antacid and Anti-Ulcer Suspension Containing Herbal Drugs. Biomed Pharmacol J 2010;3(1). Available from: http://biomedpharmajournal.org/?p=1103 |
Introduction
Gastric ulcer affects about 60% of the adults and about 80% of the child population in the tropical countries. Gastric and duodenal ulcers, gastrooesophageal reflux disease are the gastrointestinal disorders sharing a common abnormality. The therapy for these disorders is directed at the correction of an apparent imbalance between the acid and pepsin activity and the mucosal resistance. The success of the therapy is measured in terms of ulcer healing, symptom control, relapse rate etc. Herbal compounds have been used for a variety of the disorders of the gastrointestinal tract. The herbal drugs are considered to be safe for use, easily available at cheaper cost and produce minimal side effects. The herbs Glycyrrhiza glabra, Terminalia chebula, Terminalia belerica, Emblica officinalis and mineral Turbinella rapa are reported in classical ayurvedic texts to possess anti-phlogistic activity, astringent activity and acid neutralization activity that is desirable for the treatment of gastric ulcer. Glycyrrhiza glabra, which is known as the yashtimadhu in Ayurveda, is reported to increase the mucus secretion, inhiiting the enzymes that degrade the gastrocyto protective prostaglandins E and Fα thus raising their concentration. Terminalia chebula, Terminalia belerica, Emblica officinalis are tannin containing compounds.These have a property of forming a complex with the proteins. This complex has been proved to be resistant to the proteolytic enzymes.These properties that these drugs possess and the strategies,that have been put forth, for the treatment of gastric ulcers prompted for the preparation of polyherbal formulation containing the above said herbs and minerals .
Methods and Materials
Vegetable material and extract preparation
The herbal drugs like Glycyrrhiza glabra Linn, Terminalia chebula, Terminalia belerica and Emblica officinalis were procured from NKCA Pharmacy; Mysore.Turbinella rapa was obtained from Ashwini Oushadalaya, Mysore.
Preparation of triphala extract
Accurately weighed about 100g of the herbs Terminalia Chebula,Terminalia belerica and Emblica officinalis, passed individually through 120# mesh separately and macerated separately with 95% of ethyl alcohol for 4 days. Then it was filtered and the filtrate was evaporated to dryness (at a temperature not to exceed 50ºC) when gummy mass was obtained. It was further dried at a temperature not to exceed 50ºC in a vacuum drier. The powder obtained was passed through 120#. Then the equal quantities of the powder obtained from these herbs were mixed to get the triphala extract.
Preparation of triphala powder
The individual powders of the (Terminalia Chebula,Terminalia belerica and Emblica officinalis) herbs after passing through 120# was mixed in mortar to give triphala powder.
Preparation of suspension
Suspensions were prepared by using the triphala powder and triphala extract in the concentration of 2% as shown in Table 1.
Table 1: Formulation Chart of the Suspension
| 1. Ingredients | Quantity in g/ 100 ml
|
|||||||
| FP 1 | FP-2 | FP-3 | FP-4 | FE-1 | FE-2 | FE-3 | FE-4 | |
| Glycyrrhiza glabra extract | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Triphala Churna powder | 2.0 | 2.0 | 2.0 | 2.0 | – | – | – | – |
| Turbinella rapa | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 |
| Triphala Churna Extract | – | – | – | – | 2.0 | 2.0 | 2.0 | 2.0 |
| Xanthan Gum | 0.20 | 0.25 | 0.30 | 0.35 | 0.20 | 0.25 | 0.30 | 0.35 |
| Sorbitol 70% liquid | 35.0 | 35.0 | 35.0 | 35.0 | 35.0 | 35.0 | 35.0 | 35.0 |
| Distilled water to make(ml) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Preservative (methyl paraben) | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Color | q.s | q,s | q.s | q.s | q.s | q.s | q.s | q.s |
| Flavor (Menthol) | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 |
Evaluation
Thin layer chromatography
TLC provided for the entire drug in the monographs includes the identification of the drug based on its major chemical constituents as markers. Silica gel GF254 plates of uniform thickness were prepared and activated at 105ºC for 30min. and the plates were spotted with the drug extract using capillary tube. The spotted plates were dried at R.T. The solvents used were of analytical grade. Solvent system was prepared and the chromatographic chamber was saturated for 30 min. The plates were placed in the chamber and left for the development. Using different reagents did visualization of the spots and Rf values were calculated.

Evaluation of the suspension
Particle size distribution and Physico-chemical characterization such as pH measurement, Viscosity, Ease of redispersibility, Sedimentation Volume (VS), Acid Neutralization Capacity, and Stability studies were carried out.
Evaluation of Anti-ulcer activity studies
Antiulcer activity studies on the formulations were carried out on albino rats of either sex weighing 200-250 g. The Histopathological studies were performed to ascertain the ulcer prevention. The rats were randomly divided into four groups, each group containing six animals. Group 1 (Normal group) received only distilled water, Group 2 (Control group) ulcer was induced, Group 3&4 were administered with the test formulation (FP-3 & FE- 2) at the dose of 0.2 ml for seven consecutive days. Then the animals were fasted overnight. The fasted rats of groups 2, 3, 4 were administered 1ml of absolute alcohol. After 1 hr of alcohol administration, all the rats were anaesthetized and sacrificed by cervical dislocation. The stomachs were removed, and opened along the greater curvature, washed with normal saline and observed under microscope for the presence of the ulcers. The ulcers were scored as recorded in table 2:
Mean ulcer score for each group was determined and ulcer index (UI) was calculated as under:

Histopathological Studies
The tissue samples were fixed in 10% buffered formalin and processed with paraffin wax. For this study 5mm sections were stained with haematoxylin and eosin. The extent and depth of the ulceration and hemorrhage were evaluated.
Table 2: ulcer score
| 0 | Normal colored stomach. | 0.5 | Red coloration. |
| 1 | Spot ulcer | 1.5 | Hemorrhagic streaks |
| 2 | Ulcers equal to 3mm but less than 5mm | 3 | Ulcers greater than 5 mm |
Statistical Analysis
Statistical Analysis was carried out by using one-way ANOVA followed by Duncan’s multiple comparison tests. All the results obtained in the study were compared with each group. P values than 0.05 were considered statistically significant.
Table 3: TLC Identification of the Individual Herbs
| HERBAL DRUGS | RF Value | |
| Obtained | Literature | |
| 1) Glycyrrhiza glabra | 0.45 | 0.46 |
| 2) Terminalia Chebula | 0.66 | 0.68 |
| 3) Terminalia belerica | 0.70 | 0.68 |
| 4) Emblica officinalis | 0.69 | 0.68 |
Stability studies
Stability studies were carried out on the selected formulations taking gallic acid as the marker compound for 90 days at two temperatures i.e. 30 ºC /65% RH and 40 ºC / 75% RH. The samples were withdrawn at regular intervals for 30 days for a total period of 90 days. HPTLC studies were performed to ascertain the stability data.
Results And Discussion-
Identification (Figure 1) of the Individual Herbs was carried out using TLC and the standard values were obtained.
 |
Figure 1 |
Physicochemical Characterization of the suspension–
From the particle size distribution data (figure2), it can be concluded that the suspension is a coarse dispersion with particle size predominantly lying between 50-75 micrometers.
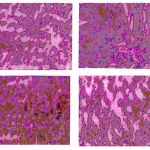 |
Figure 2 |
The results (table 4) shows the following details, that is, the pH of all the formulations lies around 8.0, which is desirable for an antacid suspension. The viscosity of the suspension containing herbal extracts is more than those containing herbal powders which may be attributed to the fact that the herbal extracts being sticky in nature contribute some value to the viscosity of the suspension. It can also be concluded that, as all the formulations are characterized by high sedimentation volume, the suspension may be flocculated suspension. The formulations FP-2, FP-3 FE-1 and FE-2 have good redispersibility with good consistency. The suspension containing herbal powders and 0.3% concentration of Xanthan gum shows the optimum quality of the suspension with regards to the consistency and redispersibility, while in case of suspension containing herbal extracts, 0.25% concentration of the Xanthan reveals the same. It is evident that the acid neutralization capacity of all the formulations lies between 2-3 mEq/ml. The minimum acid that has to be neutralized by an antacid should be 12 mEq-40 mEq and hence 5ml of the above formulations satisfies the requirement.
Table 4: Various physical parameters of the formulations
| Formulation code | Parameter | ||||
| PH (mean±S.D) | Viscosity in Cps (mean±S.D) | Sedimentation volume | Redispersibility | Acid neutralization capacity (mEq/ml)
(mean±S.D) |
|
| FP-1 | 8.02±0.14 | 29.9±0.35 | 0.88 | 2 | 2.77±0.1058 |
| FP-2 | 8.22±0.06 | 40.76±0.5 | 0.91 | 3 | 2.81±0.2551 |
| FP-3 | 8.17±0.12 | 50.73±0.4 | 0.93 | 3 | 2.613±0.3395 |
| FP-4 | 8.07±0.12 | 58.1±0.70 | 0.96 | 4 | 3.0±0.1453 |
| FE-1 | 8.04±0.13 | 39.5±0.95 | 0.90 | 3 | 2.4523±0.1102 |
| FE-2 | 8.06±0.12 | 61.6±0.1 | 0.92 | 3 | 2.38±0.4888 |
| FE-3 | 8.25±0.10 | 69.7±0.60 | 0.95 | 4 | 2.55±0.4093 |
| FE-4 | 8.12±0.15 | 78.269±1.877 | 0.98 | 5 | 2.31±0.1769 |
Standard deviation, n=3
Anti-ulcer activity
Data (table 5) showed that the Suspension containing the herbal extracts decreased in the ulcer index as compared to the one containing the fine powders of these drugs.
Table 5: Ulcer Index
| GROUP | ULCER INDEX | |
| Control | 1.01 ± 0.68 | |
| Ulcerated | 8 .68 ±1.51 | |
| Treated (FP-3) | 3.57± 2.14* | |
| Treated (FE-2) | 2.11±1.07* | |
Standard deviation, n=6, P < 0.05 as compared to the ulcerated group, and * indicates statistically significant.
Histopathological studies
The figure 3a indicates the normal mucosa of the stomach with mucus and presence of chief cells. The figure 3b indicates the ulcerated mucosa with prominent loss of mucus, and chief cells. The figure 3c indicates the pretreated mucosa with suspension containing herbal powders that shows less loss of mucus and chief cells. The figure 3d indicates the mucosa pretreated with suspension containing herbal extracts with near normal appearance to that of normal mucosa.
Stability Studies
The results of table 6 reveals that the HPTLC data indicate that there are no interactions between the ingredients, during the stability studies. The TLC data of the stability studies do not show any secondary spots, which indicates that there is no degradation-taking place during stability studies. The data of acid neutralization capacity of the suspension, which was evaluated during the stability studies, indicates that there is no change in the parameters.
Table 6: Stability studies data showing the % Gallic acid estimated by HPTLC method and the TLC data.
| Formulation |  Visited 11,862 times, 1 visit(s) today
|






