G. Mohandass1*, K. Suguna Devi2 and Amalorpavaraj3
1Sathyabama University, Chennai, India.
2Prince Dr K. Vasudevan College of Engineering and Technology, Chennai India.
3St. Joseph’s College Autonomous, Trichy, Tamil Nadu India.
Abstract
In the present study agnuside was isolated from Vitex agnus castus by bio-assay guided fractionation and subjected to antifungal assay. Agnuside showed potent activity against tested fungi namely E. floccosum (31.25 µg/ml), T. simii (62.5 µg/ml), C. lunata (250 µg/ml), T. rubrum (62.5µg/ml), C. albicans (125 µg/ml) and Scopulariopsis sp (500 µg/ml). This study report the potency of agnuside as antifungal agent developed from plant source.
Keywords
Agnuside; antifungal; dermatophytes; MIC
Download this article as:| Copy the following to cite this article: Mohandass G, Devi K. S, Amalorpavaraj. Antifungal Activity of Vitex Agnus Castus-In Vitro Study. Biomed Pharmacol J 2010;3(1) |
| Copy the following to cite this URL: Mohandass G, Devi K. S, Amalorpavaraj. Antifungal Activity of Vitex Agnus Castus-In Vitro Study. Biomed Pharmacol J 2010;3(1). Available from: http://biomedpharmajournal.org/?p=1371 |
Introduction
Pathogenic fungi, dermatophytes have the ability to invade keratinized tissues of animals, humans and cause a disease, dermatophytosis, which is the commonest human contagious fungal disease (Esquenazi et al 2004; Sidat et al 2006). The antimicrobial properties of certain Indian medicinal plants were reported based on traditional use (PerumalSamy et al 1998, 1999), and a few attempts were made on inhibitory activity against certain pathogenic fungi. Due to the increasing development of drug resistance in human pathogens as well as negative effects of certain antimicrobial agents, there is a need to search for new antifungal agent without toxicity and side effect. In this study we focused on the in vitro screening of antifungal activity of Vitexagnuscastus (agnuside) against dermatophytes and opportunistic pathogens.
Material and Methods
Plant Material
Leaves of Vitexagnuscastus were collected from botanical garden, University of Madras, Maduravoil (Chennai, Tamil Nadu, India) during June-July 2007. A specimen was deposited at department herbarium, Sathyabama University. Collected plant material was air-dried under shade at room temperature, ground with an electric grinder into fine powder and stored in airtight containers.
Table 1: Antifungal activity of isolated compounds (MIC, µg/ml)
| Tested Fungi Agnuside Flu |
| Trichophytonmentagrophytes 66/01 – <12.5 |
| Epidermophytonfloccosum 73/01 31.25 <12.5 |
| T. simii 110/02 62.5 <12.5 |
| Curvularialunata 46/01 250 <12.5 |
| Aspergillusniger MTCC 1344 – <12.5 |
| Botrytis cinerea – NT |
| T. rubrum MTCC 296 62.5 <12.5 |
| Magnaporthegrisea >500 NT |
| C. alicans 125 <12.5 |
| Scopulariopsis sp. 101/01 500 <12.5 |
| NT- Not test; Flu- Fluconazole (standard) |
Extraction and isolation
Air-dried powdered of V. agnus (1.5 kg) was extracted with ethyl acetate (2 x 2l) for 48 h at room temperature (± 25oC). The ethyl acetate crude extract was filtered and evaporated under reduced pressure. The total concentrated (160 gm) was chromatographed on a silica gel column (Merck 70–230 mesh, 800 gm, 3.5 i.d.x60 cm) and successively eluted with stepwise gradient of hexane-ethyl acetate system (0%, 5%, 10%, 20%, 30%, 50%, 70% and 100%). Eleven fractions were collected and each fraction was spotted on a precoated Silica gel 60 F254, 0.25mm thick TLC plate (Merck) and eluted in hexane: ethyl acetate (4:6) and fractions with similar Rf values in TLC pattern were pooled together. Fraction-7 (7.3 g) showed signiûcant antibacterial (MIC) and DPPH free radical inhibition. For further separation bioactive substance was chromatographed on a silica gel column and eluted with a stepwise gradient of Isoproponal alcohol–methanol (8:2) solvent system, and an active isolate of 3.5 g was obtained. This active isolate was subjected to spectral analysis. 1 H and 13 C NMR spectra were recorded with a JEOL 300MHz FT NMR spectrometer (H1) 75, MHz (13C) and chemical shifts were given in ppm. IR spectra were taken on a Perkin Elmer FT-IR spectrophotometer and mass spectra on a JEOL GC-MASS spectrometer.
 |
Figure 1: TLC of Agnuside (Single spot) |
Phytochemical analysis
The presence of phytochemicals alkaloids (Draggendorff’s), flavonoids (Shibat’as reaction), saponins (Frothing test), tannins (5% ferric chloride), terpenoids (2,4-dinitro-phenyl hydrazine), glycosides (Fehling’s solution), steroids (Liebermann’s Burchard test) were evaluated according to the methods described by Edeoga et al. (2005).
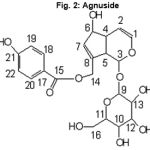 |
Figure 2: Agnuside |
Fungal strains
The following fungi were used for experiments:Trichophytonrubrum MTCC 296, T. mentagrophytes 66/01, T. simii 110/02, Epidermophytonfloccosum 73/01, Scopulariopsis sp. 101/01 Aspergillusniger MTCC 1344, Botyritiscinerea,Curvularialunata46/01, Magnaporthegrisea and Candida albicans MTCC 227.
Preparation of fungal spore
The filamentous fungi were grown on Sabouraud Dextrose Agar (SDA) slants at 28oC for 10 days and the spores were collected using sterile doubled distilled water and homogenized. Yeast wasgrown on Sabouraud Dextrose Broth (SDB) at 28oC for 48 h.
Antifungal assays
The antifungal activity was performed according to the standard reference method (NCCLS, 2002). The extracts were dissolved in 2% dimethyl sulfoxide (DMSO). The initial concentration of extract was 1mg/ml. The initial test concentration was serially diluted two-fold. Each well was inoculated with 5µl of suspension containing 104 spore/ml of fungi. The antifungal agent Fluconazole was included in the assays as positive controls; the plates were incubated for 24h up to 9 days at 27°C for dermatophytes strains. MIC was defined as the lowest extract concentration, showing no visible fungal growth after incubation time.
Results
Isolation of active compound
One active compound was obtained by using bio-assay guided fractionation (Fig. 1). Structural determination of this compound was done using different spectral technique and confirmed as agnuside (Fig. 2). It had IR absorptions at 3429 (br, hydroxyl), 1700 (Carbonyl), 1637 (olefinic region) and 1274 (C-O) Cm-1. 1H NMR (CDCL3, 300 MHZ) spectrum showed the peaks at 6.88-7.88 ppm, which corresponds to the aromatic proton. The peak appears in the range in between 3.40 to 5.20 ppm assigned to carbon connected to oxygen atom. 13 C NMR spectrums showed the peaks at 115.85 to 162.52 ppm correspond to the aromatic carbon. The carbon connected oxygen atom appears in the range between 62.52 to 96.25 ppm. The carbonyl peaks are assigned to 165.23 ppm. The EI-MS (70 eV) of the compound showed molecular ion peaks at 464 (M) +.
Phytochemical analysis
Phytochemical analysis revealed the presence of triterpenoids, flavonoids and glycosides
Antifungal assay
Agnuside showed potent activity against tested fungi (Table 1) namely E. floccosum(31.25 µg/ml), T. simii(62.5 µg/ml), C. lunata(250 µg/ml), T. rubrum(62.5µg/ml), C. albicans(125 µg/ml) and Scopulariopsissp(500 µg/ml).
Discussion
Screening of antimicrobial activity provided the required preliminary observation to select crude plant extracts with potentially useful properties for further chemical and pharmaceutical investigation.
Conclusion
Results of the present work indicate that the plant species assayed possess antifungal properties. This explains the use of these plants in folk medicine for the treatment of various diseases whose symptoms might involve fungal infections, and underline the importance of the ethno botanical approach for the selection of plants in the discovery of new bioactive compounds. We found significant antifungal activity of agnuside of Vitexagnus-castus (leaves).
References
- Edeoga, H.O., Okwu, D.E., and Baebie, M., Phytochemical constituents of some Nigerian medicinal plants. Afri.J. Biotechnol. 4: 685-688 (2005).
- Esquenazi, D., Alviano, C.S., DeSouza, W., Rozental, S., The influence of surface carbohydrates during in vitro infection of mammalian cells by the dermatophyteTrichophytonrubrum. Research in Microbiology, 155: 144-153 (2004).
- National Committee for Clinical Laboratory Standards., Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M38-A, Wayne, Pa (2002).
- PerumalSamy, R., Ignacimuthu, S., and Sen, A., Screening of 34 Indian medicinal plants for antibacterial properties, J. Ethnopharmacol., 62: 173-181 (1998).
- Perumalsamy, R., Ignacimuthu, S., Patric Raja, D., Preliminary screening of ethnomedicinal plants from India. Journal of Ethnopharmacology, 66: 235-240 (1999).
- Sidat, M.M., Correia, D., Buene, T.P.,
Tineacapitis among rural school children of the district of Magude in Maputo province, Mozambique. Mycoses,49: 480-483 (2006).







