Sanjeev Kumar Shukla*, Shubhra Shukla and Jose Mathew
Department of Biotechnology, Bundelkhand University, Jhansi India.
Corresponding Authior E-mail:sanjeevcloning@gmail.com
Abstract
Avian egg whites are a rich source of protein inhibitors of proteinases. This work describes the use of different high resolution techniques to study egg white proteins from avian species. In addition to their nutritional importance, egg white proteins present multiple functional properties, such as foaming, emulsification and heat-setting. The egg whites of 20 different avian species or varieties were examined by specific biochemical and chemical analyses, by chromatographic separations of the constituent proteins, and by examinations of the properties of several of the purified proteins. The constituents studied included sulfhydryl groups, sialic acid, lysozyme, apoprotein-flavoprotein, conalbumin, and ovalbumin. Large differences were found in the amounts of these substances in the whites from the different birds. In the cases of lysozyme and sialic acid, differences as great as 30-fold were found. Differences in the properties of several of the purified proteins were also found. The significances and values of the results from the standpoints of comparative and genetic biochemistry were discussed.
Keywords
Egg white protein; Lysozyme; Poultry species
Download this article as:| Copy the following to cite this article: Shukla S. K, Shukla S, Mathew J. The Relative Biochemical Studies of Avian Egg White Proteins. Biomed Pharmacol J 2009;2(2) |
| Copy the following to cite this URL: Shukla S. K, Shukla S, Mathew J. The Relative Biochemical Studies of Avian Egg White Proteins. Biomed Pharmacol J 2009;2(2).Available from: http://biomedpharmajournal.org/?p=761 |
Introduction
The homologous proteins from the egg whites of different avian species offer excellent opportunities for the study of the comparative and genetic biochemistry of proteins. Chicken eggs constitute one of the major protein sources of our diet1. Comparative studies of avian egg whites have utilized electrophoretic, immunological, and specific biochemical analyses. This makes egg white an essential ingredient for the food industry2. These studies have all shown significant, and in some instances large, differences between the whites from eggs of different avian species. The principal and more extensive studies, however, have relied on electrophoretic examinations of the whites3. Although these have supplied distinctive patterns which have been valuable in considering genetic aspects, in many cases they are difficult to interpret in terms of the recognized constituents of chicken egg white. Nevertheless, despite their nutritional and technological importance, hen egg white proteins are surprisingly less studied than other protein sources such as milk proteins or soya proteins. Moreover, egg white proteins from other less known poultry species, such as quail, duck, pheasant are proving to be very useful and could, in some cases,present certain advantages over hen egg white proteins4,5.
Chicken eggs are a popular and highly nutritious food, and eating raw eggs is common in many parts of the world6, 7, 8, 9. A multistate surveillance conducted in the USA between 1995 and 1996 found that as many as 50% of the responders ate undercooked or raw eggs, and more than 60% did not wash hands after cracking raw eggs, and this habit has remained unchanged for years10,11.
The present study was initiated for the purpose of obtaining more specific biochemical and chemical information on the constituents of the egg whites of a variety of avian species. The specific biochemical and chemical analyses which have been developed for chicken egg white have been employed. These analyses provide information on the presence and relative amounts of the biological activities and chemical groups as found in chicken egg white. They thus give information which is not provided by electrophoretic analyses. In addition, certain of the whites have been fractionated and the purified constituents have been partially characterized. Data have been obtained on the following constituents: ovalbumin, conalbumin, ovomucoid, lysozyme, and sialic acid. Important differences have been found for all constituents. The ovomucoids (inhibitors of proteolytic enzymes) were found to exhibit complex differences, varying both quantitatively and qualitatively in their activities, and have been recently described elsewhere.
Experimental Procedure
Procurement of Eggs
The eggs of the chicken, duck, turkey, and guinea fowl were obtained from the poultry farm, and those of the pigeon and goose were obtained from other local sources. All eggs were refrigerated within 24 hours after being laid. The eggs were usually separated within 2 days of refrigeration, and the whites were blended and stored in the frozen state until used.
Analyses
Specific chemical and biochemical methods of analysis for the components of chicken egg white as routinely used in this laboratory were employed for analyses of the other egg whites. These include the following methods: sulfhydryls by the spectrophotometric method of Boyer; flavoprotein-apoprotein by the residual and total riboflavin-binding capacity; conalbumin by the chromogenic capacity with iron; lysozyme by the lytic activity against Micrococcus lysodeikticus employing an automatic recorder; avidin by its biotin binding capacity in the yeast growth assay. The respective purified chicken egg white proteins were used as standards in the methods above. The percentages of each protein in the individual whites in this paper, therefore, indicate the amounts based on the properties of chicken egg white proteins rather than absolute amounts. Crystalline sialic acid prepared from Escherichia coli was used for the standard of sialic acid. Bound sialic acid, as it occurs in the egg whites, was liberated by heating with dilute acid or incubating with the enzyme neuraminidase.
Fractionation of Egg Whites and Physical Analyses
Fractionations of the various egg whites on CM-cellulose and DEAE cellulose were performed essentially as described for chicken egg white. Further details are given below. Sedimentation analyses were performed with a Spinco model E ultracentrifuge. Paper electrophoretic analyses were performed with a horizontal strip apparatus. Free boundary electrophoretic analyses were performed with Atto portable electrophoresis apparatus. The indices of egg whites and egg yolks were determined as previously described.
Results
General Physical Characteristics
Table I contains a general summary of the quantitative data obtained on eggs from 20 different species or varieties. Eggs varied in weight from less than 10 g to over 110 g and the whites in content of dry matter from 105 mg per ml to 115 mg per ml. In addition to the large variation in size, the eggs also varied in shape and color. General descriptions of these external physical characteristics are available from many sources. Despite the large differences in external physical characteristics, however, the physical structures of the internal contents of the eggs were quite similar. The color of the egg whites varied from clear or slightly chalky to light yellow, as usually found in chicken egg white. As will be discussed below, this difference in color is apparently related to the content of riboflavin. All eggs had thick and thin egg whites which existed in proportions that were similar to those found in fresh chicken eggs, i.e. 50 to 60% thick egg white and 40 to 50% thin egg white. Chalazae existed in all egg whites, although their apparent sizes and opacities varied considerably. For example, the chalaza in the large cassowary egg was nearly transparent and sometimes difficult to observe and could have been classified as merely a slightly more dense and thicker portion of the thick egg white. Other empirical physical characteristics, such as the yolk and white indices, were in general very similar. For example, the yolk and white indices of several cassowary eggs were 0.35 and 0.11, respectively. Average yolk and white indices of a series of chicken eggs were 0.44 and 0.07, respectively. (These indices are the numerical ratios of the heights to the widths when the broken-out egg is placed on a flat surface.
General Chemical Fractionations
The fractionations of egg whites of several avian species including the duck, turkey, goose, red jungle fowl, golden pheasant, cassowary, francolin and painted quail were performed employing CM-cellulose and DEAE-cellulose. Elutions of the proteins from the exchangers were performed employing stepwise changes in pH which had been established to give a separation of chicken egg white into its major constituents. It was possible to select intervals of pH which were sufficiently broad to allow for small differences in the isoelectric points of the homologous proteins of the different whites. Initially, the intervals of pH for elution of the proteins from CM-cellulose were at pH 4.3, 5.0, 7.0, 9, 0, and 9.0 plus 1 M NaCl. Separations were obtained in all instances, and the respective fractions contained the following proteins as indicated by specific chemical or biochemical assays: unadsorbed at pH 4.3, ovomucoid and flavoproteins; from pH 4.3
to 5.0, ovalbumin; from 5.0 to 7.0, conalbumin; from pH 7.0 to 9.0, avidin and unidentified proteins; and from pH 9.0 to 9.0 plus NaCl, lysozyme. These fractions included varying amounts of unidentified proteins depending upon the particular fraction and the species. In most instances further fractionation with smaller intervals of pH (including essentially zero gradient or starting-buffer development in some cases) for elution from CM-cellulose or with use of DEAE-cellulose was necessary to obtain proteins which were homogeneous by paper electrophoretic analyses. These were performed at pH 6.9 with 0.1 T/2 potassium phosphate buffer for 16 hours at 8 milliamperes. In some cases, however, homogeneous proteins were not difficult to obtain. Several of the ovomucoids appeared homogeneous (II), and the lysozymes of the turkey and the duck were easily crystallized and were found homogeneous. The corresponding proteins from the various whites are of course, not eluted under exactly the same conditions as those from chicken white. Variation in the elution pH values from CM-cellulose for some of the isolated ovomucoids from various whites has been reported. Even here, variation of the several ovomucoid components in a given species may be greater than from species to species. Slight differences have also been noted in the optimal pH for elution of ovalbumin of the various species from CM-cellulose. However, preliminary observations would indicate that the major proteins of all the whites were eluted from CM-cellulose within approximately 0.2 to 0.4 pH unit of the pH values at which the corresponding proteins from chicken egg white are eluted.
Lysozymes
Lysozyme activities varied from amounts which were essentially undetectable in the Psittaciforme egg white to as high as 4.2% in the red jungle fowl egg white. These large differences in lysozyme activity were not correlated with any other characteristics with the possible exception of the amount of turbidity occurring on dilutions of the egg white with water. Egg whites containing smaller amounts of lysozyme generally gave lower turbidities when diluted with 5 volumes of water or dilute buffer as previously reported in comparisons of chicken and duck whites. The lysozymes of turkey and duck white were isolated by the use of CM-cellulose and further purified by crystallization. Patterns similar to electrophoretic patterns are obtainable with gradient elution schemes. Their properties were compared with chicken lysozyme. All three formed crystals which were in the form of needles and were grossly similar. Specific enzymatic activities were the same within the limits of the error of the enzyme assay as performed in two assays (±l0%). The isoelectric points were all >pH 9.0 as determined by paper electrophoretic studies and only single peaks were observed in each case. Ultracentrifugal examinations also gave similar homogeneous patterns for the three lysozymes.
Conalbumin
The concentration of conalbumin in the various whites did not show the relatively large differences found for lysozyme activity. However, the absolute differences were much greater for conalbumin than for lysozyme. In no cases were any eggs encountered which contained obvious amounts of the iron complex of conalbumin as judged by the absence of any obvious amounts of a salmon-pink color. Limited studies indicated that the conalbumins from the different species had very similar structures and properties. One series of experiments performed directly on the whites showed that the absorption spectra of the iron complexes were essentially the same for the following whites: chicken, red jungle fowl, turkey, Gray’s francolin, and pea fowl. These were determined from 430 mµ to 510 mµ at intervals of 10 mµ. Purified preparations of these were found to have chromogenic capacities with iron within 80 to 100% of that of crystalline chicken conalbumin and to be homogeneous by free boundary electrophoresis. The isoelectric points were in the same general range as that of chicken conalbumin but slight differences in mobilities were found. Fig.1 presents patterns obtained for chicken conalbumin and mixtures containing 1% chicken conalbumin and 0.5% cassowary, turkey, or golden pheasant conalbumin at pH 4.7. The cassowary conalbumin migrated slightly faster and the turkey and golden pheasant conalbumins migrated slightly slower than the chicken conalbumin. In a single ultracentrifugal determination in which chicken and cassowary conalbumins were compared, similar homogeneous patterns were obtained. These were performed with 1% solutions in a buffer of 0.05 M NaCl and 0.05 M glycine at pH 8.5 and at 52,640 r.p.m. for 48 minutes. Cassowary conalbumin was also found to give practically identical results to chicken conalbumin in experiments comparing the relative resistances of the iron complex and the iron-free protein to hydrolysis by chymotrypsin and to thermal denaturation. The iron complex of cassowary conalbumin was also more stable than the iron-free protein to these two treatments.
Flavoprotein-apoprotein
The contents of flavoprotein and apoprotein varied not only as to total amounts of flavoprotein-apoprotein present but also as to the percentages existing as flavoprotein. The percentage of total flavoprotein-apoprotein varied from a low of 0.14% for the banded plover to a high of 0.9% for the, whereas the relative amounts of the total existing as flavoprotein varied from a low of 14% for the to a high of 48% for the guinea fowl. The flavoproteins were all nonfluorescent. In all cases the titrations with riboflavin to fluorescence gave sharp end points as previously reported with chicken flavoprotein. The visually observed yellow color of the whites was approximately proportional to the amount of riboflavin.
Sulfhyclryls and Ova and umins
The probable numbers of titratable sulfhydryl groups per mole of the ovalbumins were calculated from the data in Table I. These were made with the assumptions that the different egg whites contained amounts of ovalbumin similar to chicken egg white (55%), that the different ovalbumins have molecular weights similar to chicken ovalbumin (46,000 g), and that the different ovalbumins contribute essentially all the titratable sulfhydryls in the different whites as is the case with chicken ovalbumin.
Ovomucoid accounts for 10% of the protein produced by the tubular gland cells of the oviduct in laying birds12. The molecular weight ranges between 27 and 35 kD13, 14. About 20–25% carbohydrate content has been reported15. The variable carbohydrate content observed in ovomucoid preparations obtained from a single species is probably due to incomplete specificity of the biosynthetic enzymes16.
Results of these calculations are shown in Table II and are compared with the calculated number of sulfhydryl groups for the partially purified ovalbumins. In general, the number of sulfhydryl groups calculated from data on the egg white and isolated ovalbumins appeared to agree. Small differences could easily be due to the variation in the amounts of ovalbumins in the whites, variability in determination of sulfhydryl groups by the method employed, small impurities in the isolated ovalbumins, and the presence of unidentified proteins in the whites which contribute to the sulfhydryl content. In chicken white, for example, a small amount of a protein was isolated with an apparent isoelectric pH of 5.1 to 5.3 and a sulfhydryl content of approximately 66 pmoles per g. The possible presence of this uncharacterized protein in the egg whites of other species has not been investigated.
Table II also gives the average distance moved by the fastest moving component of each partially purified ovalbumin during paper electrophoretic analyses. These analyses were run for 16 hours at 8 milliamperes with potassium phosphate buffer, pH 6.9, 0.1 T/2. Certain of the ovalbumins appeared to move slightly further than chicken ovalbumin such as the duck and goose. On the other hand, the golden pheasant ovalbumin moved very slowly compared to the other ovalbumins. In addition to differences in migration on paper, the ovalbumins differed as to how well the components, those probably corresponding to chicken ovalbumin Al, A2, and A3, separated from each other. Most of the other patterns showed two distinct peaks with the exception of those for duck and goose ovalbumins which tended to smear together. Ultracentrifugal comparisons of the ovalbumins of the chicken, turkey and duck were made with 1% solutions in 0.1 T/2 potassium phosphate buffer at pH 6.9. The speed of the rotor was 52,640 and the time was 94 minutes. All three ovalbumins gave similar homogeneous patterns. The calculated 520 values were 2.8, 3.0 and 3.0 for the ovalbumins of chicken, duck and turkey respectively.
Table 1 :Composition of Avian egg whites
| Order | Avian Species | Egg
Weight (g) |
Analyses of whites | ||||||
| Common Name | Scientific Name | Dry
weight (mg/ml) |
Lysozymeb
(%) |
Conalbuminb
(%) |
Flavoproteib,c (%) | Sulfhydryl
µmol/g |
Sailic
Acid (%) |
||
| Caswinformes | Cassowary, double wattled | Casuarius aruensis | 650 | 110 | 0.5 | 10 | 0.6 | 18 | 2.3 |
| Anseriformes | Duck | Anas platyrhynchos | 60 | 132 | 1.2 | 2 | 0.3 | 25 | 0.11 |
| Goose | Anser anser | 150 | 133 | 0.6 | 4 | 0.3 | 36 | 0.12 | |
| Galliformes | Chicken | Gallus domesticus | 60 | 125 | 3.4 | 12 | 0.7 | 37 | 0.29 |
| Red jungle fowl | Gallus gallus | 35 | 115 | 4.2 | 11 | – | – | 0.31 | |
| Arizona scaled quail | Callipepla s. pallida | 11 | 115 | 2.8 | 6 | – | 36e | 0.18 | |
| Harlequin quail | Coturnix delegorguei | 8 | 111 | 3.1 | 15 | – | 33 | 0.22 | |
| Philippine button quail | Coturnix c. lineata | 4 | 117 | 4.3 | 16 | – | – | – | |
| Golden pheasant | Chrysolophus pictus | 30 | 104 | 2.6 | 13 | – | – | 0.42 | |
| Lady amherst | Chrysolophus amhersitiae | 30 | 108 | 1.9 | 16 | 0.6 | 27 | 0.37 | |
| Reeves’ pheasant | Syrmaticus reevesi | 30 | 128 | 1.4 | 11 | 0.4 | 24 | 0.32 | |
| Gray’s francolin | Pternistes leucoscepus | 30 | 129 | 1.4 | 10 | 49e | 49e | 0.22 | |
| Erckel’s francolin | Francolinus erckeli | – | 128 | 2.2 | 12 | – | – | 0.27 | |
| Helmeted guinea fowl | Numida meleagris | 40 | 134 | 2.2 | 9 | 0.48 | 34 | 0.38 | |
| Banded plover | Zonijer tricolor | – | 107 | 0.05 | 3 | 0.1 | – | 0.78 | |
| Green Java pea fowl | Pavo muticu | 110 | 126 | 2.8 | 9 | 0.4 | 42e | – | |
| Indian blue pea fowl | Pavo cristatusf | 100 | 105 | 2.3 | 10 | 0.6 | – | 0.62e | |
| Turkey | Meleagres gallopavo | 80d | 124 | 3.1 | 11 | 0.4 | 33 | 0.97 | |
| Columbiformes | Galapagos dove Pigeon | Nesopelia galapagoensis | 7
18 |
125
101 |
0.1
0.1 |
9
9 |
–
– |
24
– |
0.62
1.3 |
| Psittaciformes | Masked lovebird | Agapornis personata | – | 126 | <0.02 | – | – | – | 0.19 |
a Figures are averages of two or more determinations on whites of several eggs (usually several pooled whites from 2 or more eggs)
unless otherwise indicated.
b Figures are calculated on basis of proteins from chicken white as standards.
c Flavoprotein calculated on basis of total flavoprotein and apoprotein content.
d Egg weights not determined. These figures are estimates.
e Single determinations.
Table : 2-Properties of ovalbumins.
| S.N.
|
Species | Sulfhydryl groups/mole of ovalbumin | Migration of
ovalbumins on paper electrophoresisC |
|
| From Egg Whitesa
No./mole |
From Isolated fractionb
No./mole |
|||
| 1 | Chicken | 3.1 | 3.1 | 4.5 |
| 2 | Turkey | 2.8 | 2.4 | 4.0 |
| 3 | Guinea | 2.4 | 3.1 | 4.5 |
| 4 | Duck | 2.1 | 1.9 | 5.0 |
| 5 | Goose | 3.0 | 2.5 | 5.0 |
| 6 | Cassowary | 1.3 | 1.5 | 4.5 |
| 7 | Francolin | 3.9 | 3.2 | 4.5 |
| 8 | Painted quail | 2.0 | 1.9 | 5.0 |
| 9 | Golden pheasant | 2.4 | 2.6 | 3.0 |
a These were calculated from titrations directly on the egg whites assuming 55% ovalbumin and a molecular weight for each ovalbumin of 46,000 g.
b These were calculated assuming a molecular weight of 46,000 g.
c Figures given are the average distance moved from the origin in cm by the fastest moving ovalbumin component. Analyses were run for 16 hrs at 8 ma using potassium phosphate buffer, 0.1 T/2, pH 6.9. The homogeneities are discussed in the text.
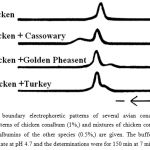 |
Figure 1: Free boundary electrophoretic patterns of several avian conalbumins. The ascending patterns of chicken conalbum (1%,) and mixtures of chicken conalbumin (1%) with the conalbumins of the other species (0.5%,) are given. The buffer was 0.1 M sodium acetate at pH 4.7 and the determinations were for 150 min at 7 milliamperes.
|
Sialic Acid
Sialic acid appeared to be bound in all the whites. Only low or insignificant amounts of sialic acid were found without preliminary hydrolysis by acid or by enzyme. The rapid rate of release of sialic acid from all the whites by neuraminidase might indicate a similar linkage of the sialic acid to the proteins in the various whites. In addition, the individual sialic acids prepared by enzymatic hydrolysis from the whites of chicken, cassowary, turkey, goose and guinea fowls had identical RF values when chromatographed on paper in butanol-acetic acid-water (4: 1: 5) and in propanol-butanol-water (1:2:1). In chicken egg white, approximately one-half of the sialic acid occurs associated with the ovomucin fraction. An approximately one-fourth greater content of sialic acid in the thick white compared to the thin white correlates with the distribution of ovomucin between these two structures.
Avidin
The biotin-combining activity of the whites of the chicken, turkey, duck and goose have been examined basis of a content of 0.05% of avidin in chicken white, turkey, duck and goose egg whites contained 0.15, 0.02, and 0.005%, respectively.
Discussion
Studies on the proteins of different species provide information useful to the taxonomist and geneticist and also to the protein chemist who may be able to relate differences in structure to differences in properties. Several workers have suggested the value of comparative compositional studies on egg whites. The following speculation from their electrophoretic studies. “It seems possible that the physiochemical character of an egg retains more of its incipient phylogeny than the more superficial aspects of the bird’s adult morphology.” Those proteins with important functional properties might be expected to undergo fewer structural changes during the evolutionary process. In avian species, the albumen provides the developing embryo with nutrients and water while limiting microbial proliferation. Egg white proteins act synergistically to contribute to antimicrobial activity. Ovotransferrin is implicated in iron-dependent bacterial inhibition 17, 18. Enhanced binding of iron to ovotransferrin is favoured in the presence of sodium citrate and/or sodium bicarbonate and leads to inhibition of bacterial growth. Although no definite functions have yet been found for the egg white proteins, they possess unique antimicrobial and antienzymatic activities.
Two types of lysozyme activity: c-type (chicken-type) and g-type (goose-type) have been described. These two lysozymes have similarities in their tertiary structures although their amino acid sequences are entirely different and antibodies directed against c-type lysozyme do not cross-react with g-type lysozyme and vice versa19, 20, 21.
The comparatively large differences in the contents of lysozyme and sialic acid (>30 fold in several instances) demonstrate the interrelationships that may be encountered. These two substances alone could provide the basis for an exhaustive study. They both can be rapidly determined in egg white by relatively simple methods. Differences in the amounts of the individual egg white proteins have been found to exist between different strains of chickens. The formation of umbiliculus, a connection between yolk sac and white, enables white proteins to move into yolk sac22. The interaction between enzyme and cystatin is close enough to exclude molecules as small as iodoacetate from the reaction and is formed with minimal conformational adaptation of either protein22, 23, 24.
These differences, however, have been small compared to the differences found between species. The possibly even greater general importance would be the comparisons of the fundamental structures of the homologous proteins from the different whites. Studies of the comparative properties and structures of homologous proteins from the different whites should also prove an important tool in understanding the relationship of structure to the properties and functional activities of the proteins. This general area has recently been receiving considerable attention in studies on ribonuclease, hemoglobin and several of the proteins or peptides with hormonal activity. The recent discovery that the ovomucoids vary quantitatively and qualitatively in their activities suggests the possible value of this approach. In like manner, the results of this and a previous study have indicated that ovalbumins with different numbers of sulfhydryl groups occur. Lysozyme should receive particular attention because of its comparative ease of preparation and its enzymatic activity.
Acknowledgments
Authors are thankful to J.C. Bose Institute of Life Science, Department of Biotechnology, Bundelkhand University, Jhansi, 284128 (U.P.), India, for providing financial support and lab facilities for this study.
References
- Powrie, W.D. and Nakai, S., Egg science and technology. Macmillan London,
61-90 (1986). - Mine, Y., Recent advances in the understanding of egg white protein functionality. Trends in Food Sci. and Technol., 6 : 225–232 (1995).
- Desert, C., Gfflerin-Dubiard, C., Nau, F., Jan, G., Val, F. and Mallard, J., Comparison of different electrophoretic separations of hen egg white proteins. J. Agricul. Food Chem., 49 : 4553–4561 (2001).
- Valuev, I.L., Valuev, L.I. and Plate, N.A., New possibilities for biospecific chromatography. Appl. Biochem. and Microbiol., 39 : 422–425 (2003).
- Hytonen, V.P., Laitinen, O.H., Grapputo, A., Kettunen, A., Savolainen, J., Kalkkinen, N., Marttila, A.T., NordLund, A.R., Nyholm, T.K.M., Paganelli, G. and Kulomaa, M.S., Characterization of poultry egg-white avidins and their potential as a tool in pretargeting cancer treatment. Biochemical J., 372 : 219–225 (2003).
- Doorduyn, Y., Van Den Brandhof, W.E., Van Duynhoven, Y.T., Wannet, W.J. and Van Pelt W. Risk factors for Salmonella Enteritidis and Typhimurium (DT104 and non-DT104) infections in The Netherlands: predominant roles for raw eggs in Enteritidis and sandboxes in Typhimurium infections. Epidemiol. Infect., 134: 617–626. (2006).
- Lievonen, S., Havulinna, A.S. and Maiala R., Egg consumption patterns and Salmonella risk in Finland. J. Food Prot., 67 : 2416–2423 (2004).
- Shiferaw, B., Yang, S., Cieslak, P., Vugia, D., Marcus, R., Koehler, J., Deneen, V. and Angulo, F., Prevalence of high-risk food consumption and food-handling practices among adults: a multistate survey, 1996 to 1997. The Foodnet Working Group. J. Food Prot. 63 : 1538–1543 (2000).
- Sin, J., Quigley, C. and Davies, M., Survey of raw egg use by home caterers. Commun. Dis. Public Health, 3 : 90–94 (2000).
- Yang, S., Leff, M.G., McTague, D., Horvath, K.A., Jackson- Thompson, J., Murayi, T., Boeselager, G.K., Melnik, T.A. and Gildemaster, M.C.. Multistate surveillance for food-handling, preparation, and consumption behaviors associated with food borne diseases: 1995 and 1996 BRFSS food-safety questions. MMWR CDC Surveill Summ 47 : 33–57 (1998).
- Fein, S., Levy, A. and Lando, A., Food Safety Survey: Summary of Major Trends in Food Handling Practices and Consumption of Potentially Risky Foods. http://www.cfsan.fda.gov/~dms/fssurvey.html. (2002).
- Palmiter, R.D., Regulation of protein synthesis in oviduct. J. Biol. Chem. 247 : 6450-6461 (1972).
- Lineweaver H. and Murray C.H., Identification of the trypsin inhibitor of egg white with ovomucoid. J. Biol. Chem.,171: 565-581(1947).
- Davis J.G., Mapes C.J. and Donovan J.W., Batch purification of ovomucoid and characterization of the purified product. Biochemistry, 10 : 39-42 (1971).
- Leach B.S., Collawn J.F., Jr. and Fish, W.W., Behaviour of glycopeptides with empirical molecular weight estimation methods. In sodium dodecyl sulfate. Biochemistry, 19 : 5734-5741 (1980).
- Melamed, M.D., Electrophoretic properties of ovomucoid. Biochem. J., 103 : 805–810 (1967).
- Valenti, P., De Stasio, A., Mastromerino, P., Seganti, L., Sinibaldi, L. and Orsi, N., Influence of bicarbonate and citrate on the bacteriostatic action of ovotransferrin towards staphylococci, FEMS Microbiology Letters, 77-79 (1981).
- Von Hunolstein, C., Ricci, M.L., Valenti, P. and Orefici, G., Lack of activity of transferrins towards. Streptococcus spp., Medical Microbiology and Immunology, 181 : 351–357 (1992).
- Hemmen, F., Mahana, W., Jolles, P. and Paraf, A., Common antigenic properties of a g-type (goose) and a c-type (duck) egg white lysozyme: antibody responses in rabbits and mice. Experientia, 48 : 79–84 (1992).
- Irwin, D.M. and Gong, Z., Molecular evolution of vertebrate goose-type lysozyme genes. Journal of Molecular Evolution, 56 : 234–242 (2003).
- Pooart, J., Torikata, T. and Araki, T., Enzymatic properties of rhea lysozyme. Bioscience Biotechnology and Biochemistry, 69 : 103–112 (2005).
- Stevens, L., Egg proteins: what are their functions? Science Progress, 79 : 65–87 (1996).
- Lindahl, P., Alriksson, E., Jornvall, H. and Bjoerk, I., Interaction of the cysteine proteinase inhibitor chicken cystatin with papain. Biochemistry, 27 : 5074–5082 (1988).
- Bjoerk, I., Alriksson, E. and Ylinenjarvi, K., Kinetics of binding of chicken cystatinto papain. Biochemistry, 28 : 1568– 1573 (1989).
- Bjork, I. and Ylinenjarvi, K., Interaction between chicken cystatin and the cysteine proteinases actinidin, chymopapain A and ficin. Biochemistry, 29 : 1770–1776 (1990).







