S. Kavitha¹, Viswanathan² and Amit Kumar Das³
1Department of Pharmacology, Krupanidhi college of Pharmacy, Bangalore India.
²Department of Pharmacology , Madras Medical College, Chennai India.
³Department of Pharmaceutical Chemistry, Krupanidhi College of Pharmacy, Bangalore India.
Corresponding Author E-mail:kushidinku@gmail.com
Abstract
The present study was carried out to evaluate the effect of polyherbal formulation (Diarun-Plus) on the blood glucose changes and nociceptive threshold and tolerance on non-diabetic mice (which rendered hyperglycemic with exogenous administration of dextrose). This study was carried in 2 phases, during phase-I, the mice were rendered hyperglycemic by repeated injections of dextrose (2g/kg i.p.) at 9,13 and 17 hours for a consecutive period of 3 days. In phase-II, the treatment period extended to 10 days similar to that of phase-I .The blood glucose level and pain threshold and pain tolerance were measured for the effect of diarun-plus on algesic state in persistent hyperglycemic mice. During phase-I (for 3 days), diarun-plus (0.5 or 1g/kg) produced mild reduction in blood glucose level, pain threshold (hind paw licking) and pain tolerance (jumping time) were found to be unaltered on fourth day. During phase II (for 10 days) blood glucose levels were lowered by diarun-plus(0.5 or 1g/kg) on 11th day,but not statistically significant. Pain threshold and pain tolerance were significantly increased on 11th day. This present study establishes the protective effect of diarun-plus in preventing the complications of hyperglycemia without inducing adverse hypoglycemia , hence this polyherbal formulation can be used an adjuvant in the treatment of diabetes mellitus associated with neuronal damage causing peripheral pain especially.
Keywords
Diarun-plus (polyherbal formulation); pain threshold; pain tolerance
Download this article as:| Copy the following to cite this article: Kavitha S, Viswanathan, Das A. K. The Effect of a Polyherbal Formulation on the Glycemic and Algesic States in Persistent Hyperglycemic in Non-Diabetic Mice. Biomed Pharmacol J 2009;2(2) |
| Copy the following to cite this URL: Kavitha S, Viswanathan, Das A. K. The Effect of a Polyherbal Formulation on the Glycemic and Algesic States in Persistent Hyperglycemic in Non-Diabetic Mice. Biomed Pharmacol J 2009;2(2).Available from: http://biomedpharmajournal.org/?p=766 |
Introduction
Diabetes mellitus is the fourth or fifth leading cause of death in most developed countries. Both IDDM and NIDDM are associated with increased mortality and morbidity due to the development of many complications such as vascular damage, neuronal damage, nephronal damage and retinal damage1 .
Earlier studies implicate hyperglycemia as a primary factor responsible for neuropathy observed in DM patients, hence pain is important sequeale of diabetic neuropathy,a varying degree of alteration in pain sensitivity is considered responsible for the complications.
This study was carried out in a non-diabetic model because diabetogenic agents such as streptozotocin and alloxan would cause neuronal damage which cannot be extended2, thus the assessment of anti nociception may not be accurate in the experimental models. Neuropathic pain is a terrible suffering experienced by chronic DM patients, a wide variety of analgesics starting from paracetamol to anti depressants are employed to alleviate the neuropathic pain without much success.
A combination of such authenticated herbs Diarun-Plus is widely recommended by practitioners of Indian medicine as an adjuvant therapy in DM patients with main emphasis to prevent the complications. Some recent studies have indicated the ability of this herbal combination to maintain a euglycemic state in experimental animals subjected to both hyperglycemia and hypoglycemia by physiological means3. It has been of interest to study the effect of this herbal combination in pain during hyperglycemic situations since neuropathic pain is one of the major complications of DM, resulting from hyperglycemia.
Hence in the persistent hyperglycemia by repeated administration of dextrose exogenously in non-diabetic mice and to investigate an alteration in the pain threshold or pain-tolerance in the animals. Further, the effect of this herbal combination on the blood glucose and the nociceptive response in these animals have been also included in this protocol.
Neuropathy in a major complication of untreated diabetes mellitus(DM). The pain experienced by these patients is not easily amenable to conventional treatment . The present study has made an attempt to find out the role of herbal combination on the possible altered pain status during hyperglycemic situation
Materials and Methods
Experimental animals
Randomly bred adult male swiss albino mice weighing between
20-25 gm obtained from (Institute Guindy) were used for the study. They were housed in Polypropylene cages in groups of three in the department animal house in normal temperature ranging between 28°-30° C. Water and food (pellet feed from TamilNadu, Veterinary Sciences department) were available ad libitum. A 12h:12h light:dark cycle was maintained.
Materials used
Polyherbal formulation (Diarun-Plus) obtained from Rumi herbal, Chennai contains
Emblica officinalis-115 mg, Curcuma longa-150mg, Momardica charantia-30mg
Eugenia jambalona-30mg, trigonella foenum-graceum-30mg and Salacia reticulate-30mg. This is fine powder of this herbal formulation was made as a suspension in 1% Carboxy methylcellulose and administered orally to experimental animals.
Dextrose-5%, Glibenclamide(gift from Dr.Reddy’s Lab) and Sodium carboxy methylcellulose (Loba).
Blood glucose was measured using AMES glucometer (Bayers diagnostics Mumbai).Eddy’s hot plate(4) was used for measurement of pain threshold and pain tolerance.55˚±0.5˚C. Maximum cut-off time of 60 seconds was allowed for these responses.
Phase –I study
This phase were rendered hyperglycemic by repeated injections of dextrose (2g/kg i.p.) at 9,3 and 17 hours for a consecutive period of 3 days. The blood glucose, pain tolerance and pain threshold were monitored on first day prior to any treatment and on fourth day morning .Diarun-plus was administered 30 minutes prior to dextrose in doses of 0.5g or 1g/kg orally (morning and evening).A separate group of animal received diarun-plus only in a dose of 1g/kg twice a day for three days. The control group of animals received 0.2ml of 1% CMC suspension orally. Each group consisted of 6 animals.
Phase-II study
In this phase the treatment period was extended to 10 days. Similar to the procedure described for phase-I study. Standard group received 10mg/kg orally as a suspension in 1% CMC for 10 days 30 minutes prior to dextrose (2g/kg i.p.) treatment. Control group receives 1%CMC 0.2ml for 10 days. Diarun-Plus of (0.5 and 1g/kg) doses were employed during this phase for 10 days.Dextrose-2g/kg i.p. was administered 3 times a day. Blood glucose level, pain-tolerance and pain-threshold were monitored on day-1 prior to the treatment and on day-11 after treatment.
The doses of diarun-plus were selected from traditional practitioner and also on previous published data
Statistical analysis
The data were subjected to statistical analysis by employing student’s’ test. A ‘P’ value of less than 0.05 was considered for statistical significance.
Results
In phase-I study, continuous administration of dextrose showed no significant increase in blood sugar level, vehicle treated group showed no changes but diarun-plus treated group showed mild insignificant reduction in blood glucose level (Table1,2,Fig1,2) instead of increasing blood glucose level compared to dextrose treated group. Similarly no significant alteration in hind paw licking time and jumping time
(Table3, Fig3) on first and fourth day in animals received both dextrose and diarun-plus.
Table 1: Changes in Glucose Level in Diarun-Plus Treated Mice After 3 Days Dextrose Administration.
| Treatmenta g/Kg | Blood Glucose Level mg/dl | |
|
Vehicle Diarun-Plus-1 Dextrose-2 Dextrose+Diarun-plus-0.5 Dextrose+Diarun- Plus-1 |
Day-1 | Day-4 |
|
128.0 ± 8.1 132.6±6.5 120.8±8.6 146.3±8.9
133.5±5.5
|
125.3 ± 6.2 128.1±7.2 134.5±8.9 127.3±7.2
122.8±4.8 |
|
a The animals received dextrose (2g/kg) i.p. at 9,13 and 17 h. Diarun-plus was administered orally twice a day 30 min prior to dextrose.
Each value represents the mean ±SEM of six observations
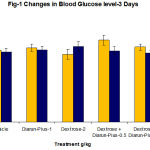 |
Figure 1: Click here to View figure |
Table 2: Changes in Hind Paw Licking(Hot Plate) in Diarun-Plus Treated Mice After 3 Days Dextrose Administration.
| Treatmenta g/Kg | Paw Licking Time (Seconds) | |
|
Vehicle Diarun-Plus-1 Dextrose-2 Dextrose+Diarun-plus-0.5 Dextrose+Diarun- Plus-1 |
Day-1 | Day-4 |
|
8.2 ± 1.3 8 ± 0.7 8.2±0.6 7.3±0.9
8.7±7.1
|
7.7 ± 0.8 8.8±1.1 9.3±0.5 7.8±0.5
10.5±0.5 |
|
a The animals received dextrose (2g/kg) i.p. at 9,13 and 17 h. Diarun-plus was administered orally twice a day 30 min prior to dextrose.
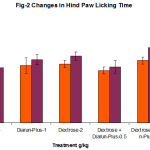 |
Figure 2: Click here to View figure |
Each value represents the mean ±SEM of six obser
Table 3: Changes in Jumping(Hot Plate) in Diarun-Plus Treated Mice After 3 Days Dextrose Administration.
| Treatmenta g/Kg | Jumping Time (Seconds) | |
|
Vehicle Diarun-Plus-1 Dextrose-2 Dextrose+Diarun-plus-0.5 Dextrose+Diarun- Plus-1 |
Day-1 | Day-4 |
|
29.8 ± 1.3 30.9 ± 2.2 30.3±1.6 30.7±1.6
25.3±7.1
|
31.3 ± 2.8 32.5±1.9 31.3±2.7 32.8±1.4
26.5±1.2 |
|
a The animals received dextrose (2g/kg) i.p. at 9,13 and 17 h. Diarun-plus was administered orally twice a day 30 min prior to dextrose.
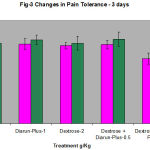 |
Figure 3: Click here to View figure |
Each value represents the mean ±SEM of six observations Phase-II : The blood glucose level did not show any significant variation between Day-1 and day-11 in animals that received either vehicle or diarun-plus 1g/kg alone (Table-4, Fig-4). Animals which received continuous dextrose for 10 days showed significant increase in blood glucose level, prior administration of diareun-plus-0.5 or 1g/kg in these animals showed significant attenuation of this hyperglycemic response on day-11 compared to day-1, however this reduction was not statistically significant. Similarly glibenclamide (10mg/kg) significantly attenuated increased blood glucose level by dextrose administration.
Table 4: Changes in Blood Glucose Level in Diarun-Plus Treated Mice After 10 Days Dextrose Administration
| Treatmenta g/Kg | Blood Glucose Level mg/dl | |
|
Vehicle
Diarun-Plus-1
Dextrose-2
Dextrose+Diarun-plus-0.5 Dextrose+Diarun- Plus-1 Dextrose+Glibenclamide |
Day-1 | Day-11 |
|
125 ±11.6
135.8±10.3
158.8±2.5
154.7±11.3
166.2±11.4
161.2±8.9 |
133.3± 7.1
131.2±6.4
229.7±13.0*
134.7±7.0
143.3±10.0
126.5±7.5 |
|
a The animals received dextrose (2g/kg) i.p. at 9,13 and 17 h. Diarun-plus was administered orally twice a day 30 min prior to dextrose.
Each value represents the mean ±SEM of six observations.
Each value represents the mean .
* p<0.01 compared with glucose level on day-1

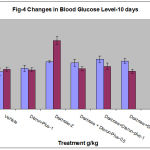 |
Figure 4: Click here to View figure |
The reaction time for hind paw licking(pain threshold) and jumping time(pain tolerance)was not significantly altered in vehicle and diarun-plus (1g/kg) alone treated animals for 10 days(Table-5, Fig-5).This reaction time was found to be reduced in both dextrose alone treated group as well as in animals which received dextrose and glibenclamide on day-11 compared to day-1.This reduction in reaction time was significantly reversed in animals that received diarun-plus (0.5 or 1g/kg) in addition to dextrose, this increased reaction time was significant compared to day-1.
Table 5: Changes in Time for Hind Paw Licking (Hot Plate) in Diarun-Plus Treated Mice After 10 Days Dextrose Administration.
| Treatmenta g/Kg | Paw Licking Time (Seconds) | |
|
Vehicle
Diarun-Plus-1
Dextrose-2
Dextrose+Diarun-plus-0.5 Dextrose+Diarun- Plus-1 Dextrose+Glibenclamide |
Day-1 | Day-4 |
|
7.8±1.0
9±0.7
9.2±0.5
7.7±0.7
7.3±0.4
10.2±1.0 |
8.7± 0.6
9±0.7
6.2±0.5**
10.7±0.7
11.5±0.9
7.3±0.6* |
|
a The animals received dextrose (2g/kg) i.p. at 9,13 and 17 h. Diarun-plus was administered orally twice a day 30 min prior to dextrose.
Each value represents the mean ±SEM of six observations.
Each value represents the mean .
* p<0.01 compared with glucose level on day-1

 |
Figure 5: Click here to View figure |
Table 6: Changes in jumping time (hot plate) in diarun-plus treated mice after 10 days dextrose administration.
| Treatmenta g/Kg | Paw Licking Time (Seconds) | |
|
Vehicle
Diarun-Plus-1
Dextrose-2
Dextrose+Diarun-plus-0.5 Dextrose+Diarun- Plus-1 Dextrose+Glibenclamide |
Day-1 | Day-4 |
|
30.3±1.6
28.8±2.3
33.8±1.9
27.5±1.6
32.0±1.8
31.3±1.2 |
32.7±1.7
31.5±1.6
26.8±1.4*
35.3±2.4●●
39.1±2.5●●*
26.2±1.6* |
|
a The animals received dextrose (2g/kg) i.p. at 9,13 and 17 h. Diarun-plus was administered orally twice a day 30 min prior to dextrose.
Each value represents the mean ±SEM of six observations.
Each value represents the mean .
* p<0.05 compared with glucose level on day-1

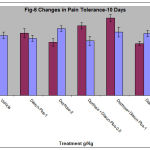 |
Figure 6: Click here to View figure |
Discussion
Diabetic mellitus is a chronic metabolic disorder that results from defects in both insulin secretion and insulin action. Type-2 diabetes is considered to be a progressive disorders and if left untreated can lead to significant morbidity and mortality.The various microvascular compications like retinopathy, neuropathy and nephropathy occurs in more than 40-50% of DM patients and macrovascular complications may lead to heart attack, stroke and peripheral vascular diseases. Tight glycemic control is believed to decrease the microvascular complications 4 , because hyperglycemia is believed to play an important role in the pathogenesis of these complications.Instead of availability of insulin, sulfonylureas and biguanides, the progressive deterioration of glycemic control could not be altered.
Inherent side effects attributable to the various class of hypoglycemic episodes, gastrointestinal disturbances hepatic injury and the major adverse effects encountered with usage of various anti-diabetics regimen. This necessitate the search for safe and effective drugs for the treatment of DM and prevention of the ensuring complications.
Indian traditional system of medicine recommends the usage of many herbs for the treatment of DM. The most popular among them are Momordica charantia, Eugenia jambolana, Gymnema sylvestre, Tinospora cordifolia, Trigonella foenum-graceum, Cynodon dactylon, Emblica officinalis, Salacia reticulate and Curcuma longa etc. in most of the patients these herbs are employed alone in combination and also as adjuvant to routine anti-diabetic therapy with modern medicine5.The common belief is the usage of herbal ingredients may help to reduce the dose of other drugs and hence limit the side effects and at the same time, these herbs are considered to prevent the various complications that develop during the course of DM6. Infact a sense of well being is the most common satisfactory reply from DM patients who receive herbal medication in addition to routine therapy. Diarun-Plus is one such herbal combination recommended by Indian traditional practitioner. Preliminary studies indicated that Diarun-Plus was able to maintain euglycemic status in experimental animals despite wide variation in blood glucose achieved by physiological means7. Besides further investigation is important for studying the effect of this herbal combination in hyperglycemic situation effects.
Chemically induced diabetic animal model in assessing the complications of DM has been questioned by many workers. Inherent neurotoxic effects produced by alloxan and streptozotocin , may lead to erroneous results in investigation of DM complication, generally neuropathy. Hence suggested an experimental model and employed physiological methods to alter the glycemic state and investigated the pain status. Pain/Allodynia is a common feature in DM patients and considered to be a consequence of diabetic neuropathy8. Moreover, hyperglycemia is considered to be the major reason for many of the neurological and vascular changes taking place during DM causing various complication9 .Thus effect of Diarun-Plus on the glycemic changes and algesic state were also taken up for investigation in this model of persistent hyperglycemia.
Diarun-Plus treatment per se for three days/ten days did not significantly alter the blood glucose level or pain threshold or pain tolerance in albino mice. But prior administration of Diarun-Plus restored the euglycemic states elicited by dextrose to euglycemic status, additionally the algesic state was also modified as evidenced by elevated levels in pain threshold and pain tolerance in the anti-nociceptive assay.
Many experimental study in animal noticed the correlation between hyperglycemia and decrease in Pain threshold, eventhough there are contradictory reports prevailing10,11, 12,13.Thus the present result showed that persistent hyperglycemic state decreases the pain threshold and pain tolerance but anti-nociceptive responses has been noted after Diarun-Plus.
Hypoglycemic effect of glibenclamide is not accompanied by an anti-nociceptive response, glibenclamide acts to release insulin by a blocking effect of K+-ATP channels . Besides it is an established fact that K+-ATP channels plays an important role in anti-nociceptive mechanism14.Further it is an established fact that K+ -ATP channel plays an important role on antinociceptive mechanism15.
The enhanced nociception in glibenclamide treated animal despite a hypoglycemic response may be attributed to the inherent potassium channel blocking action of this compound.
Attenuation of hyperglycemic response by Diarun-plus observed in the present study may be attributed to the presence of these herbs like Eugenia Jambolana, Momordica charantia, Trigonella foenum-gracius, Emblica officinalis, Salacia reticulate, Curcuma longa and Gymnema sylvestre16.In the present study pain threshold and pain tolerance were elevated after Diarun-Plus administration. This observation indicates that Diarun-Plus administration can prevent the alteration in pain sensory function due to hyperglycemia. Thus Diarun-Plus may be considered as useful adjuvant in preventing diabetic complications particularly neuropathy. This contention draws support from the report that magniferin, a xanthone isolated from Salacia reticulate (ingredient of diarun-plus) delays the onset and progression of diabetic complications17 . Further ingredients like Emblica officinalis18 , Trigonella foenum-grceum) are endowed with potent antioxidant properties. It is well established that oxidative stress per se may contribute to the development of complications of diabetes19. The antioxidant properties possessed by the ingredients present in diarun-plus may be considered to prevent the development of neuropathy like complications in DM.
This present study experimental model can be considered novel in its approach since hyperglycemia is achieved by physiological means and this hyperglycemic response has resulted in alterations in pain sensitivity indicating neuropathic changes, this may not be equated with the changes that occurs in the disease states of diabetes mellitus. Further studies in established diabetes mellitus(experimental or clinical) will be desirable in confirm these observations.
An agent that appears protection against diabetic complications without a propensity to induce adverse hypoglycemia is the most desired agent as an adjuvant in the treatment of DM. Diarun-Plus can be considered to fulfill this criterion.
Conclusion
Treatment of a progressive metabolic disorder like diabetes mellitus remains a challenge to medical scientists inspite of the availability of wide range of drugs. The use of several herbal preparation along with routine therapy is very popular all over the world, the real value of the herbal preparations which are used as adjuvants is not fully investigated. Attempts to understand the mechanism of action of these herbal supplements in curtailing the hyperglycemia and prevention of diabetic complications will be very useful in identifying novel anti-diabetic agents. The present study can be considered as a slip towards this direction.
References
- Abdel Barry J.A, Abdel-AHassan IA and AI Haheem M.H.H. “Hypoglycemic and antihyperglycemic effects of Trigonella-foenum-graecumleft in normal and alloxan induced diabetic rats” J.Ethnopharmacol, 58(3): 149-155 (1997)
- Bennett H. Peter Joslin’s Diabetes mellitus 13 th edition, Lia febiger publication, 193 (1994)
- De Fronzo R.A Pharmacologic theory for type-2 diabetes mellitus Annals of Internal medicine, 4: 359-68 (1997).
- Fox A, Eastwood C, Manning D and Urliant, “Critical evaluation of the streptozotocin model of painful diabetic neuropathy in the rat.” Pain, 81:301-316 (1999)
- Ghosh M.N: Fundamentals of experimental Pharmacology Second edition, Scientific book agency, Calcutta, 150 (1984)
- Goodman and Gilman, The Pharmacological basis of therapeutics, 9thedition, Mc Graw-Hill Medical Publishing Division, 1507.
- Horackova M and Murphy M G,”Effects of chronic diabetes mellitus on the electrical and contractile activities, fatty acid profiles and ultrastructures of isolated rat ventricular myocytes pflugers”, Arch Eur Physiol, 411: 564-572 (1988)
- Jose J.K. and Kuttan et al, Annal Res Bulletin,15: 46-52 (1995)
- Khanna P, jain S C, Panagariya A, Dixit VP, “Hypoglycemic activity of Polypeptide P from a plant source” J Nat Prod, 44:48-655 (1981)
- Kohli K.R, Singh R.H.., “ A clinical trial of Jambu (Eugenia Jambolanqa in non-insulin dependent diabetes mellitus” Journal of research in Ayurveda and Siddha, Vol-14(3-4): 89-97 (1993)
- M C Whorter L.S., “ Biological complimentary therapies A focus on botanical products in diabetes”, Diabetes Spectrum, !4(4): 199-208 (2001)
- Raz I Hardai D, Selfzer Z and Melmed RN, “ Effect of hyperglycemia on pain perception and on efficacy of morphine analgesia in rats” Diabetes , 37 : 1253-1259 (1988)
- Sarma G R K, “ An experimental and clinical study on pain sensitivity diabetes mellitus and correlation with biochemical parameters M.D dissertation Pondicherry University, (1996)
- Sathiavathy G.V, M.K Rama and Sharma M., Indian Council of Medical Research , New Delhi Vol-1(1976)
- Senthilvel G, Jagathesan M, Maheswaran E, Thiruganasambantham P and Viswanathan S, “ Effect of a Polyherbal formulationIdiarun) on the glycemic status modified by physiological means in non-diabetic mice and rats (under Publication)
- Simon G S and Deway W L, “ Narcotics and diabetes . “ The effects of streptozotocin induced diabetes on the antinociceptive potency of morphine” J Pharmacol Exp Ther, 218 : 318-23 (1981)
- Yoshikawa M. Murakami J and Matsuda H., “Medicinal food stuffs X structures of new triterpene glycosides gymnemosides-c, -d, -e and –f from the leaves of Gymnema sylvestre R.Br.: Influence of Gymnema glycosides on glucose uptake in rat small intestinal fragments”. Chemical & Pharmaceutical Bulletin, 45(12): 2038-2304 (1997)
- Yoshikawa M, Murakami t, Shimada H, Matsuda Yamahara J, Tanabe G and Muraoka O (1997), “Salacinol potent antidiabetic principle with unique thiosugar sulfonium sulfate structure from the ayurvedic traditional medicine Salacia reticulate in Srilanka and India tetrahedron letters 38(48): 8367-8370 (1997)







