M. S. Mohamed Jaabir*, A. Mansoor Hussain, T. Shariq Afsar and S. Senthil Kumar
Department of Biotechnology, Jamal Mohamed College (Autonomous), Tiruchirappalli - 620 020 India.
Abstract
Peltophorum pterocarpum (Tam: Malai porasu), Cassia auriculata (Tam: Avaram), Cassia alata (Tam: Seemaiaganthi) and Lamprachaenium microcephalum (Tam: Brahmadandi) were chosen for the study to induce apoptosis. The methanolic extracts of the leaves were dried and dissolved in DMSO in concentration of 100mg/mL for treating MCF-7 (Breast cancer) cell line grown in RPMI- C medium with 10% Foetal Bovine Serum at pH 7.1, temperature 370C and 5% CO2. The cytotoxicity of extracts on MCF-7 was determined by MTT Assay and found to be as 3mg/ml for Peltophorum pterocarpum and Cassia auriculata and 1mg/ml for Lamprachaenium microcephalum and Cassia alata. The sublethal concentrations of all the four extracts were treated and the morphology of cells after AOEB/Hoechst 33258 staining was visualized under Epifluorescence. Cells treated with Lamprachaenium microcephalum and Cassia alata showed resemblance to Apoptotic death while that of Peltophorum pterocarpum and Cassia auriculata resembled much of Necrosis.
Keywords
Peltophorum pterocarpum; Cassia auriculata; Cassia alata; Lamprachaenium microcephalum; MTT Assay
Download this article as:| Copy the following to cite this article: Jaabir M. M. S, Hussain A. M, Afsar T. S, Kumar S. S. Study on the Apoptotic Properties of Methanolic Extracts of Peltophorum Pterocarpum, Cassia Auriculata, Cassia Alata and Lamprachaenium Microcephalum. Biomed Pharmacol J 2009;2(2) |
| Copy the following to cite this URL: Jaabir M. M. S, Hussain A. M, Afsar T. S, Kumar S. S. Study on the Apoptotic Properties of Methanolic Extracts of Peltophorum Pterocarpum, Cassia Auriculata, Cassia Alata and Lamprachaenium Microcephalum. Biomed Pharmacol J 2009;2(2).Available from: http://biomedpharmajournal.org/?p=868 |
Introduction
Cancer is one of most dreadful diseases of 20th and 21st century. Although people of all ages develop cancer, most types are more common in people over the age of 50. Cancer usually develops gradually over many years, the result of a complex mix of environmental, nutritional, behavioral, and hereditary factors. Apoptosis is a fundamental physiological process in mammals in which cells die by activating an Intrinsic Suicide Mechanism1. Defects in apoptotic signaling pathways play critical roles in a multiplicity of pathophysiological status including cancer. The highly regulated systematic nature of apoptosis lends itself to distinct morphological and biochemical criteria including selective and tightly controlled activation of proteolytic cascades that result in an ordered disassembly of cells2.
Modern surgery had significantly reduced the cancer mortality rate, chemotherapy and radiation therapy were reducing the death rate not more than 5% and they are producing numerous side effects. But majority of tumor cells were resistant to these therapies therefore search for the alternative agent which can cure tumor without any side effects is under way. Among them, herbal plants are used as complementary and alternative medicine. Use of herbal medicines in Asia represents a long history of human interactions with the environment. According to World Health organization (WHO) more than 80% of the World’s population relies on traditional medicine for their primary health care. These medicinal herbs were used for long time to cure illness and some of these plants were believed to promote resistance against infection. Recently great emphasize is given towards the complementary and alternative medicine treatment. Ayurveda is one of the best complementary medicinal treatments for cancer therapy. Ayurveda describes cancer as inflammatory or non-inflammatory swellings3.
Herbal decoctions consisting of multiple herbs each possessing tremendous potential for a cancer cure are commonly used in Ayurveda. These formulations are reported to work on multiple biochemical pathways and are capable of influencing several organ systems simultaneously. The benefit of a herbal decoction is that it can nourish the body as a whole by supporting various organ systems.
In the present study, methanolic extracts of the leaves of Peltophorum pterocarpum, Cassia auriculata, Cassia alata and Lamprachaenium microcephalum have been tested for the ability to induce cell death in cancer cell line by apoptosis as determined by AOEB / HOESCHT 33258 staining and morphological examination.
Material and Methods
Plant Collection and Methanolic Extraction
Plants such as Cassia auriculata, Cassia alata, Peltophorum pterocarpum and Lamprachaenium microcephalum were collected from places around Tiruchirappalli District and identified by the Postgraduate and Research Department of Plant Biology and Plant Biotechnology, St. Joseph’s college (Autonomous), Tiruchirappalli. Leaves of the plants were dried under shade, powdered and extracted using methanol as solvent by hot extraction method with Soxhlet apparatus. The extracts were collected, dried and used for treating the cells at various concentrations4.
Cell lines
MCF-7 Cell line was derived from National Centre for Cell Science, Pune. The Cell line was cultured in RPMI- C medium with 10% Foetal Bovine Serum. The cell line was cultured at pH 7.1, temperature 370C with 5% CO2. All reagents and media were purchased from Himedia, Mumbai, India.
RPMI Complete Medium (RPMI-C)
RPMI – 1640 was supplemented with 10% heat – inactivated foetal Bovine serum (FBS), 2 mM L- Glutamine, 20mM HEPES Buffer, 100µg/ml penicillin, 150µg/ml streptomycin and 50µM 2-mercapto-ethanol.
MTT Assay
MTT (3 – 4,5 – dimethyl thiazol-2-yl) 2-5, diphenyl tetrazolium bromide), a pale yellow substrate is converted into a formazan, a violet compound by the activity of succinate dehydrogenase of mitochondria. Since the conversion takes place in living cells, the amount of formazan is directly correlated with the number of viable cells present. MTT assay was done following the method of Mosmann with slight modification5,6,7. In brief, cells (1×105 per well in 200µl medium) were seeded in 96 well plate and allowed to adhere for 24 hours at 370C with 5% CO2 in air. Medium was aspirated and replaced with medium containing methanolic extracts in DMSO in concentrations ranging from 1, 10, 100, 1000 and 10000mg/ml for 24 hours at 370C with 5% CO2 in air. Then 10µl of MTT (5mg/ml stock solution) in PBS was added to each well containing 100 µl of cell suspension and re-incubated for 4 h at 370C. The reaction mixture was carefully taken out and 200 µl of DMSO was added to each well and mixed thoroughly. After 10 minutes, the color was read at 530nm using multi-well microplate reader (BIORAD, USA). The untreated sets were also run parallel under the identical conditions and served as ‘Control’.
AOEB Staining
MCF-7 cells were cultured in RPMI-C at 37°C in a humidified atmosphere of 5%CO2 in air for 24 or 48 h in the presence or absence of each methanolic extracts of the leaves mentioned above. After treatment, 100µl of these cell suspensions (1×105 cells/ml) in RPMI-1640 were collected, centrifuged at 200g for 1 minute and mixed with 1µl of dye mix (1µg/ml acridine orange plus 1µg/ml Ethidium bromide in PBS). Observation was carried out at 400x using an epi-illumination microscope (Olympus, Japan) with a filter combination suitable for fluorescein visualization. At least 200 cells were counted and the number of cells with fragmented nuclei, increased cytoplasm and condensed chromatin, which reliably indicate apoptosis, were determined as previously described8,9,10.
Hoechst H-33258 Staining
About 1×105 cells were washed twice with PBS and then re-suspended in 500 µl of Ice cold methanol and placed at 40C for 5 minutes. Cells were washed once with PBS and resuspended in a solution of Hoescht H-33258 nuclear stain (Sigma) (1/100 dilution of Hoescht H-33258 in PBS) and placed for 5 minutes at 40C (Light protected). Samples were subsequently washed twice with PBS and finally the pellet was resuspended 20µl of PBS. Cells were spread on microscope mounting plate, covered with a cover slip and analyzed under a Fluorescence Microscope (Olympus, Japan) with emission at 492 nm with excitation at 356 nm. Nuclei exhibiting fragmentation and disintegration were observed.
Results and Discussion
Cell Viability Assay
As determined by MTT assay, the methanolic extracts of the leaves of Peltophorum pterocarpum, Cassia auriculata, Cassia alata and Lamprachaenium microcephalum strongly affected the survival of the MCF-7 cell line in time and dose dependent manner. MCF-7 cell line is a breast cancer cell line being very sensitive to cytolytic agents and is easily maintained. In the study, the cells were treated with various concentrations of the extracts ranging from 1, 10, 100, 1000 and 10,000 mg/ml and the cell viability was measured by MTT assay. The inhibition of the cell viability was clearly observed in a dose –dependent manner. The IC50 value was 3mg/ml for Peltophorum pterocarpum and Cassia auriculata and 1mg/ml for Lamprachaenium microcephalum and Cassia alata for MCR-7 after 24 hours (Table 1).
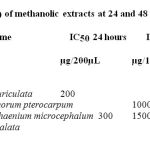 |
Table 1: IC50 of methanolic extracts at 24 and 48 Hours.
|
Analysis of Apoptosis
Acridine orange (AO) – Ethidium bromide (EB) and Hoechst staining were performed to evidence possible changes in cellular morphology. The control cells appeared green and blue respectively (Fig.1and 2), whereas methanolic extract – treated cells were shown to possess abnormalities. Microscopic images of the cells treated with Lamprachaenium microcephalum and Cassia alata revealed nuclear alterations typical of the apoptotic process. Both the stains revealed cytoplasmic blebbling, presence of apoptotic bodies, marginalization of chromatin and innumerable micronuclei in cells treated with both the methanolic extracts of Lamprachaenium microcephalum and Cassia alata (Fig 1and 2).. On the other hand, death pattern of cells treated with Cassia auriculata and Peltophorum pterocarpum was much similar to necrosis.
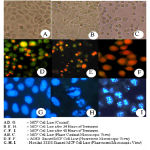 |
Figure 1: Microscopic views of MCF – 7 cell lines treated with Lamprachaenium microphalum.
|
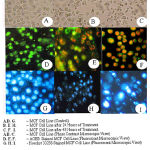 |
Figure 2: Microscopic views of MCF – 7 cell lines treated with Cassia alata.
|
Conclusion
Results from this study allow us to demonstrate that it is mainly the extracts of Lamprachaenium microcephalum and Cassia alata to inhibit tumor cells. Such inhibition may be mediated by Apoptosis. It is also further implicated from the study that the methanolic extracts of Lamprachaenium microcephalum and Cassia alata can be re-extracted with other solvent such as Chloroform, ethyl acetate and water for further analysis into the suspected apoptosis mediated cell death.
Acknowledgements
The authors are thankful to Dr. M. Sheik Mohamed, Principal, Dr. M. M. Shahul Hameed, Head, Dept. of Biotechnology, and the Management, Jamal Mohamed College (Autonomous), Tiruchirappalli – 620 020, Tamil Nadu, for their constant support and provision of the facilities.
References
- Kerr, J. F. “History of the events leading to the formulation of the apoptosis concept.” Toxicology, 181-182 (2002).
- Green, D.R. and Evam, G. L. “A matter of life and death.” Cancer Cell, 19 – 30 (2002).
- Rama Ranga, S. “A herbal medicine for the treatment of lung cancer.” Molecular and Cellular Biochemistry, 280: 125-133 (2005).
- Premalatha Balachandran and Rajagopal Govindarajan. “Cancer an ayurvedic prespective.” Pharmacological Research, 51: 19-30 (2005).
- Mosmann, T. “Rapid colorimetric assay for the growth and survival: Application to Proliferation and Cytotoxicity assays.” Journal of Immunolological methods, 65: 55-63(1983).
- Raj Upreti, K., Kannan, A. and Pant, A. B. “Experimental exposure of Arsenic in cultured at intestinal epithelial cells and cell line: Toxicological consequences.” Toxicology in Vitro, 21: 32-40 (2007).
- Mosmann. “MTT assay.” J. Immunol. Methods, 65: 55 (1983).
- Caldus Lopes, E., Garcaa, M.G., Vallon, L., Alvarez, E. and Hajos, S.E. “Correlation with decreased apoptosis and multi – drug resistance in murine leukemic T Cell lines.” Leuk. Lymphoma, 42: 775-787(2001).
- Kurz, E.U., Wilson, S.E., Leader, K.B., Sampey, B.P., Allan, W.P., Yalowich, J.C. and Kroll, D.J. “The histone deacetylase inhibitor sodium butyrate induces DNA topoisomerase II alpha expression and confers hypersensitivity to Etoposide in human leukemic cell lines.” Mol.Cancer Ther, 1: 121-131(2001).
- O’Connell, A.R., Holohan, C., Torriglia, A., Lee, B.F. and Stenson-Cox, C. “Characterization of a serine protease-mediated cell death program activated in human leukemia cells.” Experimental Cell Research, 312: 27-39 (2006).







