Manuscript accepted on :December 19, 2009
Published online on: 17-11-2015
Plagiarism Check: Yes
T. Karthiyayini¹*, Rajesh Kumar¹, K. L. Senthil Kumar¹, Ram Kumar Sahu ² and Amit Roy³
¹Department of Pharmacognosy, Padmavathi College of Pharmacy, Dharmapuri India.
²Department of Pharmacognosy, Oriental College of Pharmacy, Bhopal India.
³Columbia College of Pharmacy, Mandhar, Raipur India.
Corresponding Author E-mail:singh.chandasingh2009@gmail.com
Abstract
In order to evaluate the antidiabetic activity of powder fruit of Cucumis sativus. The different doses of ethanol extract were screened for their effects on serum glucose levels in streptozotocin-induced rats and lipid profile (biochemical parameters) in blood were also observed with histopathological studies. The oral administration of 200 and 400 mg/kg body weight of ethanol extracts of powder fruit of Cucumis sativus exhibited significant antidiabetic effects in streptozotocin induced rats as compared to standard drug. In the same study the action of the extracts on diabetes induced hyperlipidemia was analyzed where the extracts significantly lowered the elevated cholesterol as well as LDL level. The drug has the potential to act as antidiabetic as well as antihyperlipidemic. The study revels that ethanol extract at dose of 400 mg/kg body weight exhibited more significant antidiabetic activity.
Keywords
Antidiabetic; Lipid profile; Cucumis sativus; Strepozotocin; Glibenclamide
Download this article as:| Copy the following to cite this article: Karthiyayini T, Kumar R, Kumar K. L. S, Sahu R. K, Roy A. Evaluation of Antidiabetic and Hypolipidemic Effect of Cucumis Sativus Fruit in Streptozotocin-Induced-Diabetic Rats. Biomed Pharmacol J 2009;2(2) |
| Copy the following to cite this URL: Karthiyayini T, Kumar R, Kumar K. L. S, Sahu R. K, Roy A. Evaluation of Antidiabetic and Hypolipidemic Effect of Cucumis Sativus Fruit in Streptozotocin-Induced-Diabetic Rats. Biomed Pharmacol J 2009;2(2).Available from: http://biomedpharmajournal.org/?p=829 |
Introduction
Diabetes mellitus has affected a considerable population and in the future it will be a major disorder affecting people across the globe, irrespective of sex, age and socio-economic status. Insulin has proved to be effective to some extent in increasing the life expectancy of diabetic patients, but is not a permanent solution since there are many drawbacks of this therapy. Also the therapy with oral hypoglycemic agents is not satisfactory. Thus, the search for a new therapeutically agent devoid of adverse effect originating from plants used in traditional medicine would be of interest.
The Cucumis sativus (Cucurbitaceae), it is known as cucumber, is a creeping vine that roots in the ground and grows up trellises or other supporting frames, wrapping around ribbing with thin, spiraling tendrils. The plant has large leaves that form a canopy over the fruit. The fruit is roughly cylindrical, elongated, with tapered ends, and may be as large as 60 cm long and 10 cm in diameter (1, 2). The main objectives of this study was to assess the antidiabetic potential of ethanol extracts of powdered fruits of Cucumis sativus in control of blood glucose levels and effectiveness on various biochemical parameters viz total cholesterol, triglycerides (TGL), high density lipoprotein, (HDL), low density lipoprotein, (LDL), very low density lipoprotein, (VLDL), with histopathological studies.
Materials and Methods
Plant material
The fruits of Cucumis sativus has been collected from Dharmapuri district of Tamil Nadu, India. The specimen for the present research was identified as Cucumis sativus by Dr. P. Jayaraman, Botanist, Plant Anatomy Research Centre (PARC), Chennai.
Preparation of Cucumis sativus fruits ethanolic extract
The powder of fruits (500gm) of Cucumis sativus, was packed well in Soxhlet apparatus and extracted with ethanol until the completion of the extraction. The extract was filtered while hot, and the resultant extract was distilled in vacuum under reduced pressure in order to remove the solvent completely and dried in a desiccator (3).
Animals
Male wistar albino rats having weight 170-220gm were kept in quarantine for 10 days under standard husbandry conditions (27.3o, Relative humidity 65 ±10%) for 12 hrs in dark and light cycle respectively and were given standard food and water ad. libitum. The study was permitted by the Institution Animal Ethical Committee.
Acute toxicity testing
Acute toxicity testing was performed for ethanol extract following OECD guidelines-420, fixed dose Procedure. Where a fixed dose level of extract starting from 50, 100, 200, 500, 1000, increasing upto 2000mg/kg body weight was given, sign and symptoms of toxicity were observed for next 48 hrs. No toxicity or death was observed in experimental rats (4, 5).
Oral glucose tolerance test (OGTT)
The oral glucose tolerance test (6) was performed in overnight fasted (18 h) normal rats. Rats divided into three groups (n = 6) were administered drinking water, ethanol extract of 200 mg/kg and 400 mg/kg, respectively. Glucose (2 g/kg) was fed 30 min prior to the administration of extracts. Blood was withdrawn from the retro orbital sinus at 30, 60 and 120 min of extract administration and the plasma obtained after centrifugation at 3000 rpm was estimated for fasting plasma glucose levels using a glucose oxidase–peroxidase glucose estimation kit.
Induction of non-insulin dependent Diabetes mellitus (NIDDM)
NIDDM was induced (7,8) in overnight fasted adult Wistar strain albino male rats weighing 170–220 gm by a single intraperitoneal injection of 60 mg/kg streptozotocin , 15 min after the i.p. administration of 120 mg/kg of nicotinamide. Streptozotocin (STZ) was dissolved in citrate buffer (pH 4.5) and nicotinamide was dissolved in normal saline. Hyperglycemia was confirmed by the elevated glucose levels in plasma, determined at 72 h and then on day 7 after injection. The threshold value of fasting plasma glucose to diagnose diabetes was taken as > 126 mg/dl. Only those rats were found to have permanent NIDDM were used for the study.
Experimental design
The animals were segregated into five groups of six rats each. The extract was administered for 12 days. Group I served as normal control rats administered drinking water daily for 12 days; Group II diabetic control rats administered drinking water daily for 12 days; Group III diabetic rats administered ethanol extract of 200 mg/kg body weight; Group IV diabetic rats administered ethanol extract of 400 mg/kg body weight; Group V diabetic rats administered standard drug Glibenclamide (0.25 mg/kg) for 12 days. The fasting glucose levels were determined on day 1, 5 and 12 of extract administration.
Estimation of biochemical parameters
The biochemical parameters were determined on day 12 after the animals were sacrificed by cervical dislocation. Total cholesterol, triglycerides (TGL), high-density lipoprotein (HDL), low-density lipoprotein (LDL), very low-density lipoprotein (VLDL), by Glucose oxidase method using auto-analyzer (9, 10).
Histopathology
All the animals were sacrificed on 12th day by cervical dislocation, pancreases were isolated and were subjected to histopathological studies and microscopical findings were noted.
Statistical Analysis
The results are expressed as mean ± SEM of six independent experiments. Statistical significance between group was evaluated by one-way analysis of variance (ANOVA) followed by Dunnet’s test. A P < 0.05 value was considered as statistically significant.
Results and Discussion
During study it was found that, significant elevation in blood glucose in diabetic control group as compared with normal animals at the end of the 12-day experimental period. Comparing with diabetic group different doses of ethanol extracts significantly lowered the elevated blood glucose levels. When ethanol extract of 200 mg/kg and 400 mg/kg body weight of Cucumis sativus were administered to glucose loaded normal rats fasted for 18 h, significant reduction in plasma glucose level was observed after 30 min. The extracts reduced plasma glucose level to normal at 90 min (Table 1).
Table 1: Effect of ethanol extract of Cucumis sativus on oral glucose tolerance test
| Group | Plasma glucose concentration (mg/dl) | ||
| 30 min | 60 min | 90 min | |
| Normal control | 72.55±1.95 | 97.79±2.07
|
95.49±2.17 |
| Normal + Ethanol Extract (200 mg/kg) | 69.42±1.19 | 91.17±1.73 | 80.55±2.51* |
| Normal + Ethanol Extract (400 mg/kg) | 66.11±2.15* | 86.82±2.58* | 75.05±1.86* |
Values are expressed as mean ± SEM (Number of animals, n=6); * Significantly different from the normal control at P<0.05
As expected, the administration of streptozotocin induced significant hyperglycemia at blood glucose level >200mg/dl in rats. This hyperglycemia was significantly (P<0.05) lowered by the administration of 200 mg/kg and 400 mg/kg body weight ethanol extract and Glibenclamide (Table 2) after oral administration. Furthermore, 400 mg/kg body weight ethanol extract showed more significant antidiabetic activity.
Table 2: Effect of ethanol extract of Cucumis sativus on fasting plasma glucose level in rats.
| Group | Fasting plasma glucose concentration (mg/dl) | ||
| Day 1 | Day 5 | Day 12 | |
| Normal control | 82.21±1.43 | 84.87±1.87 | 81.48±1.83 |
| Diabetic control (Streptozotocin) | 199.4±1.62* | 212±2.29* | 236.6±2.80* |
| Diabetic + Ethanol Extract(200 mg/kg) | 197.4±3.34* | 164±2.61* | 131.1±3.22* |
| Diabetic + Ethanol Extract(400 mg/kg) | 198.5±2.0* | 127.9±3.87* | 96.47±3.27* |
| Diabetic + Standard Glibenclamide (0.25 mg/kg) | 200±2.69* | 102.9±3.01* | 80.90±1.14 |
Values are expressed as mean ± SEM (Number of animals, n=6); * Significantly different from the normal control at P<0.05
The effect of the extracts on diabetes induced hyperlipidemia was also studied. It was observed that due to diabetes there was an increase in the total cholesterol levels as well as triglyceride levels. The HDL levels were reduced in the diabetic animals and the VLDL and LDL levels were increased significantly (Table 3).
Table 3: Determination of biochemical parameters after treatment with ethanol extract of Cucumis sativus and Glibenclamide.
| Group | Total cholesterol (mg/dl)
|
HDL (mg/dl)
|
Triglycerides (mg/dl)
|
VLDL
|
LDL
|
| Normal control | 84.20±2.91 | 54.41±2.69 | 85.35±2.91 | 34.4±2.69 | 43.56±3.64 |
| Diabetic control (Streptozotocin) | 154.43±4.42* | 51.13±1.97 | 182.5±3.08* | 68.7±3.69* | 160.3±4.43* |
| Diabetic + Ethanol Extract(200 mg/kg) | 116.8±4.71* | 50.15±2.71 | 114.7±3.62* | 45.55±1.84* | 93.67±4.53* |
| Diabetic + Ethanol Extract(400 mg/kg) | 86.56±3.05* | 56.75±2.86 | 95.47±3.36* | 35.36±2.51 | 63.97±2.31* |
| Diabetic + Standard Glibenclamide (0.25 mg/kg) | 60.75±2.53* | 48.6±3.15 | 90.99±3.01 | 31.54±1.92 | 38.52±2.42 |
Values are expressed as mean ± SEM (Number of animals, n=6); * Significantly different from the normal control at P<0.05
Both the doses extracts showed a significant decrease in the total cholesterol levels and triglyceride levels. In particular, the ethanol extract of 400 mg/kg body weight showed a much relevant action. It also increased the HDL level and was successful in suppressing the VLDL and LDL levels as compared to the standard drug (Table 3).
The histopathological study of normal group (Fig. 1a) showed a normal pancreatic structure. The pancreas presents in the group of animals treated with ethanol extract (Fig. 1c and Fig. 1d) showed more or less similar appearance of pancreatic lobules, acing and cells as compared to standard drugs (Fig. 1e), whereas pancreas of diabetic control animals showed partially damaged or even destroyed pancreatic lobules, acing, cells, islet size, shaped and number (Fig. 1b).
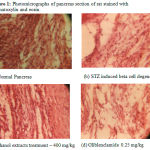 |
Figure 1: Photomicrographs of pancreas section of rat stained with haematoxylin and eosin.
|
From these results, it can be concluded that the ethanol extract of the fruits of Cucumis sativus possess antidiabetic as well as antihyperlipidemic action against streptozotocin induced hyperglycemia. These results seem to confirm the alleged antidiabetic activity by the traditional medicine.
References
- Renner, S.S., Schaefer, H, Kocyan, A., “Phylogenetics of Cucumis (Cucurbitaceae): Cucumber (C. sativus) belongs in an Asian/Australian clade far from melon (C. melo)”, BMC Evolutionary Biology, 7:58; 1-11 (2007).
- http://www.ayushveda.com/herbs/cucumis-sativus.htm.
- Sharma, S.B., Nasir, A., Prabhu, K.M., Murthy, P.S. Dev, G., “Hypoglycemic and hypolipidemic effect of ethonolic extract of seed of Eugenia jambolana in alloxan-induce diabetic rabbits” Journal of Ethanopharmacology, 85 (2-3); 201-206 (2003).
- Ecobichon, D.J., “Fixed Dose Procedure Guidline 420” The Basis of Toxicity Testing, 2nd edition, CRC Press, 1997.
- Ghosh, M.N. In: Fundamentals of Experimental Pharmacology, Scientific Book Agency, Calcutta, 1984.
- Shriwaikar, A., Rajendran, K., Punitha, I.S., “Antidiabetic activity of alcoholic stem extract of Coscinium fenestratum in Streptozotocin-nicotinamide type 2 diabetic rats” Journal of Ethnopharmacology, 97; 369-374 (2005).
- Angel, I., Burcelin, R., Girard, J. , Salomon, Z., “Normalization of insulin secretion by selective alpha-2 adrenoceptor antagonist receptors GLUT-4 glucose transporter expression in adipose tissue of type- II diabetic rats” Endocrinology, 137; 2022–2027 (1996).
- Pellegrino, M., Christophe, B., Rene, G., “Development of a new model of type II diabetes in adult rats administered with Streptozotocin and nicotinamide” Diabetes, 47; 224 (1998).
- Nyarko, A.K., Sittie, A.A., Addy, M.E., “The basis for the antihyperglycaemic activity of Indigofera arrecta in the rat” Phytotherapy Research, 7; 1–4 (1993).
- Barham, D., Trinder, P., “An improved color reagent for the determination of blood glucose by the oxidase system” Analyst, 97; 142-145 (1972).







