Manuscript accepted on :November 06, 2009
Published online on: 20-11-2015
Plagiarism Check: Yes
A. Julius
Department of Biochemistry, Sree Balaji Dental College, Bharath University of Higher Education and Research, Narayanapuram, Pallikaranai, Chennai – 600 100 India.
Abstract
In this study the biochemical changes were observed in the vector control workers who involve themselves in the control of vectors and vector borne diseases. The vector control workers were prone to two major risks one is the parasite inoculation and the other is exposure to the toxic chemicals what they spray.
Keywords
Diphenyl dichloro trichloroethane (DDT); Malathion; Anopheles culcifacies; Anopheles Stephensi
Download this article as:| Copy the following to cite this article: Julius A. Biochemical Changes in Vector Control Workers. Biomed Pharmacol J 2009;2(2) |
| Copy the following to cite this URL: Julius A. Biochemical Changes in Vector Control Workers. Biomed Pharmacol J 2009;2(2). Available from: http://biomedpharmajournal.org/?p=1030 |
Introduction
The vectors of malaria in India. are Anopheles culcifacies, Anopheles Stephensi, Anopheles fluviatilis, Anopheles sundaicus, Anopheles philippinensis, Anopheles minimus and Anopheles balabacensis. Anopheles culcifacies is widely distributed throughout the plains of India which is responsible for spreading the malaria.. Malarial parasites are transmitted from infected people to susceptible people by the bite of female Anopheles mosquitoes. Vector mosquitoes become infected by feeding on the blood of infected people and the parasite undergo another phase of reproduction in the infected mosquito. India is endemic to vector-borne diseases requiring spraying insecticides (Sharma, 1985). The spraying of organic poisonous pesticides would be effective in the control of vectors. DDT was used in large quantities. Total DDT residue in blood in exposed workers is reported to be 10 times higher than the same in unexposed control (Chand et al., 1991). Serum organochlorine residues are reported at a higher concentration in the workers engaged in spraying DDT, HCH, and lindane for vector control in Sao Jose de Riopreto, Brazil (Minelli and Riberiro,1996). A.culcifacies the vector responsible for the spread of malaria developed resistance to DDT hence control of vector and malaria was not effective. This was confirmed by the susceptibility tests for insecticides. As replacement insecticides, dieldrin and BHC were considered. All the chemicals used in various concentrations, all these years, nearly for a centaury are ineffective to eradicate or even control the vectors to a considerable degree till today. Thus the continuous spraying of these poisonous chemicals caused irreversible damage to living creatures, plants and animals from micro-organism to man causing irreversible damage to the environment, ecology, atmosphere, soil, subsoil, water and all the precious gift of nature.
Materials And Methods
Experimental Design
The subjects were spray men recruited in various zones of the Corporation of Chennai. Employees of the Corporation of Chennai with similar Socio-economic conditions who were not involved at any time in spraying operations formed the control group.
Haematological indices and Biochemical Picture
Haemoglobin, Packed cell volume (PCV), RBC (Red blood corpuscles ) count or erythrocyte count, Mean corpuscuscular volume (MCV), Mean corpuscular haemoglobin (MCH), Mean corpuscular haemoglobin concentration (MCHC), Osmotic fragility, and the biochemical parameters like blood glucose, serum cholesterol, urea and creatinine were estimated by the following methods.
Haemoglobin was estimated by the cyanmethhaemoglobin method of Drabkin and Austin (1932), as the standard method by the International Committee for standardization in Hematology (1965) and British Standards Institution (1966). Haematocrit [Packed Cell Volume (PCV)] was estimated by the Wintrobe macromethod (Wintrobe, 1933). Haematocrit is defined as the volume of erythrocytes expressed as a percentage of the volume of the whole blood. Erythrocyte count was estimated by the haemocytometer method (John 1972). The erythrocyte count is expressed as cells per cubic millimeter of blood. Erythrocytes indices were arrived at from the method described by Wolf et al (1973). Osmotic fragility of erythrocytes was done by the method of Wolf et al (1973). Blood glucose was estimated by the method of Dubowski (1962) modified by Sasaki and Matsui (1972). Cholesterol was determined by ferricchloride colour reaction by the method of Parekh and Jung (1970). Urea was estimated by the method of Natelson (1957). Plasma creatinine was measured by the method of Brod and Sirota (1948)
Results and Discussion
The haematological and biochemical changes in the spraymen were represented in table – 1. Haemoglobin and packed cell volume decreased in the spraymen when compared with the controls. Similarly the levels of RBC showed a significant change (P<0.05) when compared with the controls. The values of MCV and MCH also showed reduction in the spraymen. The osmotic fragility of RBC increased in the spraymen (P<0.05) which is shown in Figure – 1
Table 1: Heamatological indices in control and spraymen.
| Particulars | Control | Spraymen |
| Haemoglobin (g/dl) | 10.20±0.57 | 8.05±4.08* |
| Packed cell volume (%) | 33.62±4.78 | 27.15±11.72* |
| RBCCount(x10 cells/cu.mm of blood) | 4.20±0.25 | 4.05±1.08* |
| MCV (µµm) | 80±21.38 | 67.03±28.02* |
| MCH (µµg) | 24.28±7.1 | 19.87±6.83* |
| MCHC(%) | 30.35±0.78 | 29.65±0.78 |
| Osmotic Fragility | 0.40±0.01 | 0.46±0.006* |
| Glucose (mg/dl) | 99.29±19.32 | 76.16±15.35* |
| Cholesterol (mg/dl) | 188.76±23.77 | 162.41±27.58* |
| Urea (mg/dl) | 32.09±8.33 | 22.98±14.82 |
| Creatinine (mg/dl) | 1.14±0.47 |
1.32±0.55 |
Values are expressed as mean ±
Represents significance at the range of p<0.05
Osmotic fragility curves of Control and Spraymen
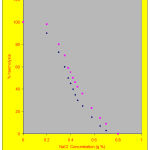 |
Figure 1:
|
The levels of glucose and cholesterol were decreased significantly in spraymen (P<0.05) when compared with the controls. Creatinine and urea were within the range suggesting normal function of kidney. A sublethal dose of karate was administered rabbits have shown significant increase in the total erythrocyte count and PCV along with SGOT and SGPT after 15 days of administration (Shakoori et al 1992). The low levels of glucose suggests hypoglycemia in these spraymen. . Feeding dietary pirimiphos- methyl, an insecticide to rats for 28 days has produced hypoglycemia with increased blood urea and and increased the excretion of urea, protein, glucorinic ucid, and ethereal sulphate in urine (Rajini and Krishnakumari, 1988). Joshi et al (1996) observed low cholesterol level (150 mg %) in 38 % of spraymen. A significant association is observed between their length of exposure, their levels of cholesterol and the HCH isomers in the blood of spraymen.
The spraymen with longer duration of spraying generally had one or more of the following clinical symptoms . The early symptoms observed were the headache, giddiness, vertigo, nausea, vomiting, blurred vision, sweating, constriction of pupils, excessive lacrimation and salivation. They also showed weakness, tightness in chest, non -reactive pin point pupils, diarrhea, abdominal cramps, bleeding from gums, nose or skin, fatigue, dizziness and chest pain. More than 95 % of the spraymen were alcoholics and smokers. Chronic toxicity is present, where the effects are produced by long term intake of lower or intermittent doses (Sharp 1986)
Reference
- Sharma V.P., Malaria problems of pollution and prospects of integrated disease vector control in India. Regional meeting of the national MAS. Committees of Central and South Asian Coutries, New Delhi, India. (1985).
- Chand B., Sankaranarayanan T., Yadava R.L., Narasimham M.V., J.Commun.Dis. 23 (4) : 245 – 247 (1991).
- Minelli E.V., Reberiro M.L., Department of Organic Chemistry, Institute of Chemistry, UNESP C.P.355 : 14800 -900 (1996).
- Drabkin, D.L.R. and Austin, J.H. J. Biol. Chem.,98:719 – 733. (1932).
- Wintrobe. Am.J. Med. Sci., 135:58 (1933).
- John, M.B. In : Laboratory Medicine Haematology, Fourth Edition, C.V.Mosby Co., St. Louis pp.1198 – 1204. (1972).
- Wolf, P.F., Ferguson, P., Mills. I.T., Von Der Muehll, E, and Thompson, M, (ed), John Wiely and Sons, Inc., New York, pp. 250 – 252. (1973).
- Dubowski, K.M.Clin.Chem., 8 : 215 – 235 (1962).
- Sasaki, T. and Matsui, S. rinsho Kagaku, 1: 346 – 358 (1972).
- Parekh, A.C.and Jung, D.H. Anal. Chem., 42 : 1423 – 1427 (1970). (Visited 145 times, 1 visits today)







