C. Narasimha Rao¹, K. Balaji¹, C. Narasimha Rao² and P. Venkateswarlu¹*
1Department of Chemistry, Sri Venkateswara University, Tirupati - 517 502 India.
²Department of Zoology, Sri Venkateswara University, Tirupati - 517 502 India
Corresponding Author E-mail: pvprofvenkat51@gmail.com
Abstract
A sensitive differential pulse voltammetric (DPV) method for the determination of Nalmefene(NLM) at Clay modified carbon paste electrode (CMCPE) was developed. Peak currents showed a linear response in the concentration range 1.4 × 10-9 to 5.2 × 10-5 M. The effect of pH of buffer solution, accumulation time and accumulation potential on peak currents were studied. Limit of detection (LOD) and limit of quantization (LOQ) were found to be 1.1 × 10-8 and 0.334 × 10-7 M respectively with a correlation coefficient of 0.9982. The proposed method has been successfully applied for the determination of NLM in spiked urine samples, human serum samples and pharmaceutical formulations.
Keywords
Nalmefene; Clay modified carbon paste electrode; differential pulse voltammetry
Download this article as:| Copy the following to cite this article: Rao C. N, Balaji K, Rao C. N, Venkateswarlu P. Electroanalysis of Nalmefene and its Determination in Pharmaceutical Formulations and Biological Fluid Samples. Biomed. Pharmacol. J.2009;2(1) |
| Copy the following to cite this URL: Rao C. N, Balaji K, Rao C. N, Venkateswarlu P. Electroanalysis of Nalmefene and its Determination in Pharmaceutical Formulations and Biological Fluid Samples. Biomed. Pharmacol. J.2009;2(1). Available from: http://biomedpharmajournal.org/?p=678 |
Introduction
Nalmefene [17-cyclopropylmethyl-4,5α-epoxy-6-methylenemorphinan-3,14-diol] (NLM) is an opioid antagonist used in the treatment of opioid over dose and postoperative opioid depression. GC-MS (1) and HPLC (2 ) methods were reported for the determination of NLM. But voltammetric methods have not been reported which are simple, rapid and more accurate than the above methods. We reported the determination of drugs based on their electrochemical reduction (3, 4). In the present method, a clay modified carbon paste electrode has been employed because they are able to adsorb and to incorporate electroactive species for the determination of an analyte. (5-10).
Experimental
Voltammograms were recorded with Metrohm 757 VA computrace (Herisau, Switzerland).Nalmefene (NLM) was purchased from Sigma. Graphite powder (l-2 mm particle size), paraffin oil from Aldrich India Ltd., Bangalore. All chemicals used for the preparation of buffers and supporting electrolytes are of reagent grade.
Recommended Procedure
An appropriate amount of NLM working standard solution is placed in the electrolytic cell which contained Britton Robinson buffer of pH 3.0. Subsequently a steam of oxygen-free nitrogen gas is passed into the solution for 10 min. The laboratory made working electrode is placed in the cell, during the deposition of the test species the solution is stirred for 150 s at
-0.4 V. The stirring (2000rpm) is stopped and after 25 s of equilibration time, the cathodic sweep is carried out toward negative potential. All the measurements are made at 21± 2 °C.
Results and Discussion
Cyclic Voltammetry
Fig. 1 illustrates the cyclic voltammogram recorded for NLM at carbon paste electrode (CPE) and clay modified carbon paste electrode (CMCPE). On scanning towards a negative potential on a bare carbon paste electrode, only a much smaller cathodic peak is observed. When CMCPE is used a large increase in the peak currents is observed. No peaks are observed in the anodic sweep indicating that the reduction of NLM under investigation is of irreversible.
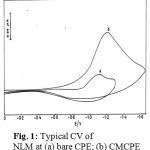 |
Figure 1: Typical CV of NLM at (a) bare CPE; (b) CMCPE.
|
Differential Pulse Voltammetry
Fig. 2 illustrates differential pulse voltammogram obtained at bare carbon paste electrode and clay modified carbon paste electrodes for NLM in BR buffer of pH 3.0. From the obtained results the peak currents obtained at clay modified carbon paste electrode are almost twice than those at carbon paste electrode of concentration 1.6×10-9M NLM.
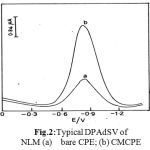 |
Figure 2: Typical DPAdSV of NLM (a) bare CPE; (b) CMCPE .
|
The peak is attributed to the reduction of carbon, carbon double bond according to the currently accepted mechanism for the electroreduction of carbon, carbon double bond containing compounds.(11, 12).
Scheme.1 Reduction of Nalmefene
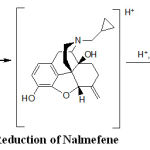 |
Scheme 1: Reduction of Nalmefene.
|
Effect of pH, Accumulation Potential and Accumulation Time
The results from the overall the experiment show that peak current is maximum at the pH 3.0. The peak currents of NLM increase as the accumulation potential increases from -0.1 V and reaches maximum at -0.4 V; as the Eacc increases further, the ip values started decreasing. Thus, an optimal accumulation potential of-0.4 V is used for further studies.Sharp increasing peak currents are obtained up to accumulation time 300 s and 150 s of NLM at CPE and CMCPE respectively. For longer accumulation times above 300 and 150 s the peak currents practically level off. . So in the further studies, a preconcentration time of 150 s is preferred as effective criteria.
Table 1: Experimental data of NLM.
| Parameters | NLM | |
| CPE | CMCPE | |
| Linearity range (M) | 2.4 ´ 10-8 to | 1.4 ´ 10-9 to |
| 1.6 ´ 10-5 | 5.2 ´ 10-5 | |
| Calibration curve equation | Y(mA)=0.3035X + 0.0335 | Y(mA)= 0.9862 X + 0.1195 |
| Correlation coefficient | 0.9975 | 0.9982 |
| L.O.D (M) | 1.6 ´ 10-8 | 1.1 ´ 10-9 |
| L.O.Q (M) | 0.533 ´ 10-7 | 0.334 ´ 10-8 |
| Repeatability of | 4.12 | 4.18 |
| peak currents | ||
| %RSD) | ||
| Repeatability of | 0.43 | 0.49 |
| Peak potentials | ||
| %RSD) | ||
| Reproducibility of peak currents | 3.51 | 3.82 |
| %RSD) | ||
| Reproducibility of potentials | 0.32 | 0.41 |
| %RSD) | ||
| Numbers of assays | 12 | 12 |
Table 2: Chosen Experimental Conditions.
| Variable | Chosen Value |
| pH | 3.0 |
| Buffer volume (ml) | 10 |
| Temperature (°C) | 21 |
| Purge time (s) | 300 |
| Accumulation potential (V) | -0.4 |
| Accumulation time (s) | 150 |
| Rest time (s) | 25 |
| Stirring rate (rpm) | 2000 |
| Scan rate (mVs-1) | 10 |
| Pulse amplitude (mV) | 50 |
Table 3: Determination of NLM in Pharmaceutical formulations.
| Name of the drug | Amount labeled (m.g/L) | *Average amount found
(m.g/L) |
Recovery percentage
(%) |
+ S.D | RSD |
| NLM | 2 | 1.956 | 97.8 | 0.0152 | 0.78 |
| 4 | 3.923 | 98.07 | 0.0602 | 1.534 | |
| 6 | 5.80 | 96.66 | 0.0670 | 1.155 |
Table 4 : Determination of NLM in spiked human serum samples.
| Name of the drug | Amount Spiked (m.g/L) | *Average amount found
(m.g/L) |
Recovery percentage
(%) |
+ S.D | RSD |
| NLM | 2 | 1.987 | 99.35 | 0.0152 | 0.765 |
| 6 | 5.957 | 99.28 | 0.0251 | 0.4213 | |
| 10 | 9.933 | 99.33 | 0.0321 | 0.323 |
Table 5: Determination of NLM in spiked human serum samples
| Name of the drug | Amount Spiked (m.g/L) | *Average amount found
(m.g/L) |
Recovery percentage
(%) |
+ S.D | RSD |
| NLM | 2 | 1.981 | 99.05 | 0.022 | 1.1 |
| 4 | 3.90 | 97.50 | 0.0353 | 0.905 | |
| 6 | 3.892 | 97.3 | 0.033 | 0.84 |
Conclusion
Higher sensitivity has achieved at CMCPE when compared to CPE. The irreversibility of reduction behavior of NLM has been investigated. The present method has been compared with already existing methods for the determination of NLM and found better recovery, more accurate, more sensitive and achieved at lower detection limits.
References
- Shan Xie, Raymond F. Suckow, Barbara J. Mason, David Allen, Thomas B.Cooper, J. Chrom. B.: Anal. Tech. Biomed. Life Sci., 773 (2002) 143-149.
- James Z. Chou, Henrik Albeck, Mary Jeanne Kreek,J. Chrom. Biomed. Appli., 613 (1993) 359-364.
- C.Narasimha Rao, M. Suman, C.Narasimha Rao, K. Balaji and P. Venkateswarlu, Material sciences research India, Vol. 5(2), 383-390 (2008).
- C.Narasimha Rao, K. Balaji, C.Narasimha Rao, and P. Venkateswarlu, Material sciences research India, Vol. 5(2), 305-312 (2008).
- Z. Navratilova and Petre Kula, Fresenius. J. Anal. Chem. 367 (2000) 369.
- P. Hernandez, J. Vivente, M. Gonzalz and L. Hernandez, Talanta 37 (8) (1990) 789.
- L. Hernandez, P. Hernandez and E. Lorenzo, “Contemporary Electroanalytical Chemistry” (Ed., A. Ivaska) Plenum Press, New York (1990) p. 205.
- Valdimir Borek and Matthew J. Morra, Environ. Sci. Technol., 32 (1998) 2149.
- Jyh-Myng Zen and Ping-Jyh Chen, Anal. Chem., 69 (1997) 5087.
- Y. Sallez, P. Bianco, E. Lojou, J. Electroanal. Chem. 493 (2000) 37.
- S. Fedez, de Bentono, J. M. Moreda, A 329 (1996) 25-31
- M. Sreedhar, Madhusudana T. Reddy, K. Balaji, Jayarama S. Reddy, Intern. J.Environ. Anal. Chem. 86 (2006) 757-767.







