Tahir M. Malla and N. Ganesh
Department of Research, Jawaharlal Nehru Cancer Hospital and Research Centre, Bhopal India.
Abstract
Heavy metals are known to be persistent in the aquatic environment and gradually accumulate and magnify through the process known as bioaccumulation and biomagnifications. Vermillion, also called as Sindoor, a traditional cosmetic of Hindu married women has lead and mercury as heavy metals that lead to toxicity in aquatic animals. Plenty of synthetic sindoor used in idol immersion finally reaches the higher levels of food chain and can be harmful. Our present study was an attempt to determine the possibility of toxicity in fresh water catfish Heteropneustes fossilis induced by the synthetic sindoor. The study revealed chromosomal aberrations and tissue toxicity in fishes treated with higher doses of sindoor. However, no such observations were noted at the lower doses
Keywords
Sindoor (vermillion); Heavy metals; Heteropneustes fossilis; metaphase; tissue toxicity
Download this article as:| Copy the following to cite this article: Malla T. M, Ganesh N. Cytogenetic and Tissue Toxicity by Synthetic Sindoor in Fresh Water Catfish Heteropneustes Fossilis. Biomed. Pharmacol. J.2009;2(1) |
| Copy the following to cite this URL: Malla T. M, Ganesh N. Cytogenetic and Tissue Toxicity by Synthetic Sindoor in Fresh Water Catfish Heteropneustes Fossilis. Biomed. Pharmacol. J.2009;2(1). Available from: http://biomedpharmajournal.org/?p=617 |
Introduction
Vermillion “Sindoor” is usually smeared in the hair parting line of Hindu married women and also used in Indian temples. It contains red mercuric oxide with lead. Plenty of commonly available synthetic sindoor is being used in the religious rituals especially during the idol immersion of Hindu’s that contains lead (Pb), mercury (Hg) and industrial dyes, that indirectly damage the human beings and other animals through the consumption of polluted water and aquatic food like fishes.1 Lead and mercury cause many adverse effects such as cancer, reproductive, liver and thyroid disorders. Fish exposed to high levels of lead and mercury exhibit a wide range of toxicity including muscular and neurological degeneration and destructive growth inhibition, mortality, reproductive problems and paralysis.2 These toxic substances may indirectly reach to the human system through the food chain. Increasing environment pollution and lack of public awareness, especially in developing countries like India, have forced scientists to study the direct and indirect effects of the disposal of industrial and other pollutants on the aquatic environment. 3
No doubt, the effects of pollutants are usually displayed first at the biochemical and molecular levels. Then, they lead to genetic changes which become cytologically visible, especially in the tissues of organisms which are good pollutant bioaccumulators, e.g. fish and molluscs.4 Mutations can be grouped as chromosome mutations, comprising changes in chromosome number and structure (i.e. chromosomal changes or aberrations) and gene mutations which can not be observed at chromosomal level, but can be determined from phenotypic changes in individual organisms. Chromosomal aberrations in some animals including fish in the wild could serve as useful indicators of the presence and action of clastogenic chemicals in areas known to be polluted with toxic chemicals or their products. 5,6
Genotoxicity can be correlated with reproductive effects such as gametic loss due to cell death, embryonic mortality and heritable mutation in a range of model animals including polychaete worms, nematodes, sea urchins, amphibians and fish.
The present study was undertaken to evaluate the cytogenetic and histological toxicity of the synthetic sindoor in the freshwater catfish Heteropneustes fossilis.
Materials and Methods
Sample collection
Healthy fishes of the average weight of 200gms were collected from Upper Lake of Bhopal, M.P. and housed in well aerated aquaria. These fishes were acclimatized for 1 month for the acquaintance of the given experimental conditions. Fishes were fed with raw beef macerated pieces and wheat dough. The selected fishes were divided in two groups.
Group (A)
The control without any treatment included five fishes.
Group (B)
Group (B) was the sindoor treated group and was further sub-divided into 9 sub-groups as per the sindoor dose treated with. Each group comprised of 4 fishes. The sindoor doses were altered to understand in depth and rationally the effect and severity of the toxicity and dose limiting factors. The treatment groups where named as H1, H2, H3, H4, H5, H6, H7, H8 and H9 treated with 50, 100, 500, 1000, 2000, 3000, 4000, 10000, 40000 ppm concentration for one month.
Cytogenetic Analysis
Prior to the dissection, the fishes were injected with 0.05% colchicine. The fishes were dissected after 1hour. The organs were collected and minced in normal saline. The minced tissue was filtered through a fine nylon mesh to get a single cell suspension. After centrifugation at 2000rpm for 10-15 minutes, the supernatant was discarded and treated with hypotonic solution – 0.56% KCl for 1 hour. The hypotonic activity was inhibited by addition of 1 ml freshly prepared chilled Cornoy’s fixative and centrifuged at 2000 rpm for 10 min. Supernatant was discarded and Carnoy’s Fixative 6-8ml was added. The cell suspension was washed by repeating the above procedure for 5 to 6 times. The slides were prepared by the air drop method and stained with 4-5% Giemsa Stain for 10-15 min. Metaphase spreads were observed under light Microscope (Olympus BX 60) under oil immersion.
Histopathological Analysis
Intestine was flushed with normal saline for 2-4 times and treated with Bouin’s fixative. The tissue was washed under running tap water till Bouin’s was removed (approx 1-2 hrs) and passed through the series. The tissues were incubated in Xylene-I and Xylene-II for 1 hour. Finally the tissue was dipped in Xylene+Wax for 1 hour. In the final process the tissues were blocked in Wax I and Wax II for 1 hr and embedded in wax blocks. The sections of 5 microns were cut by microtome and the slides were prepared. The sections were stained by a series of stains: (1) Xylene-I (Absolute-Xylene). (2) Xylene-II (Absolute-Xylene). (3) Xylene-III (Absolute-Xylene). (4) Alcohol – I (Absolute Alcohol). (5) Alcohol –II (Absolute Alcohol). (6) Hematoxyline. (7) Eosin (8) 1% acid alcohol. The slides were dipped in eosin stain 2-3 times and mount with DPX. The slides were observed under light microscope (Olympus- BX60).
Result and Discussion
The cytogenetic analysis reveals the normal diploid chromosome number i.e. 56-58 in the control group and the same chromosome number were observed till 2000 ppm doses. Two variant diploid chromosome model numbers 56 and 58 were there in H. fossilis. However, increase in the treatment dose leads to hypodiploidy. Apart from the numerical aberrations, structural aberrations mainly deletions, minutes, fragments and pulverization were also recorded at the increased treatment doses of vermillion.
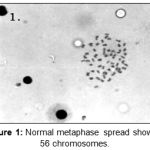 |
Figure 1: Normal metaphase spread showing 56 chromosomes.
|
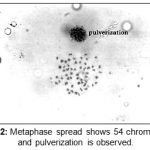 |
Figure 2: Metaphase spread shows 54 chromosomes and pulverization is observed.
|
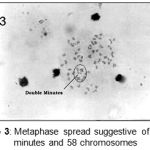 |
Figure 3: Metaphase spread suggestive of double minutes and 58 chromosomes.
|
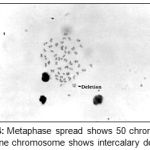 |
Figure 4: Metaphase spread shows 50 chromosomes and one chromosome shows intercalary deletion.
|
The Potential of genotoxicity biomarkers as predictors of detrimental environmental effects, such as altered chromosome number of fish must be rigorously determined. Recent research to evaluate relationships between genotoxic responses in animals is described from an ecotoxicological perspective. A study of ICPEMC stated that more than 20,000 chemicals had been evaluated for genotoxicity.7
With the above reference, our study based on the sindoor genotoxicity where the control group has its normal chromosome modal number with the variance of diploid 56 and 58. However, till 0 to 2000 ppm there was no difference in their diploid number but plate number 46, 50 revealed acrocentric association which is a direct association of two slap chromosome, belongs to group acrocentric or telocentric, finally defined as Robertsonian translocation and a single minute suggestive of terminal deletion of single chromatid at their distil end presenting as minutes respectively.
The evidence of genotoxicity is reflected and systemized from the higher dose 3000 ppm onwards and exhibited a remarkable genetic polymorphism in fish modal chromosome number where the hypodiploidy was noted. Chromosomal aberrations in fish could serve as useful indicators of the presence and action of clastogenic chemicals in areas known to be polluted with chemical products.6, 8 Many carcinogenic chemicals must be metabolically activated. Many types of DNA damage caused by mutagens present in water induce an alteration in chromosomes, so the measurement of chromosomal aberrations offers an acceptable parameter for monitoring mutagenic substances in water.
Heteropneustes fossilis has been analyzed histopathologically. The transverse section of control sample exhibited proper distribution of three layers of intestine, viz. Lamina propria, sub mucosa and mucosa. The lamina propria, sub mucosa, mucosa and specially villi had shown normal pattern in control. The proliferation compartment and maturation compartment has shown normal mitotic division and also the epithelial gland (Goblet mucous cells) and epithelial cell (Columnar cells) have shown normal distribution at the absorption compartment at lower dose of synthetic sindoor. The above observation is supported by Banerjee et al., 1993, where the lower dose of heavy metals has not shown much difference in the intestinal cells.9 However, H1 experimented with 2000 ppm presented with hyperchromoatic dysplastic cells and villus erosion. At 3000 to 40000 ppm, the tissue toxicity increased in which the goblet mucous cells lead to the exhaustion or shedding and subsequent disappearance.
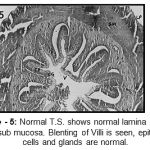 |
Figure 5: Normal T.S. shows normal lamina propria and sub mucosa. Blenting of Villi is seen, epithelial cells and glands are normal.
|
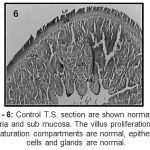 |
Figure 6: Control T.S. section are shown normal lamina propria and sub mucosa. The villus proliferation and maturation compartments are normal, epithelial cells and glands are normal.
|
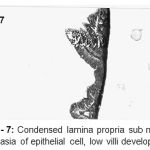 |
Figure 7: Condensed lamina propria sub mucosal, dysphasia of epithelial cell, low villi development.
|
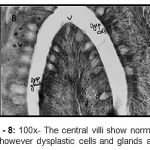 |
Figure 8: 100x- The central villi show normal villus pattern however dysplastic cells and glands are seen.
|
Conclusion
From the above discussion the genotoxicity study ruled out to understand the possible mutagenic effect caused by vermillion has been studied and found that there is no much difference in the modal chromosome number observed at lower concentration of vermillion but, produces structural changes in the chromosomes like acrocentric association, single minutes, double minutes etc. However, numerical aberration and severe nuclear damage was observed at higher doses. The above observations correlated with previous study are suggestive of genomic instability in Heteropneustes fossilis induced by synthetic sindoor. It also concludes that the lower dose 2000 ppm has not shown any tissue toxicity. However, mucosal dysplasia and villi erosion has marked a primary toxicity against vermillion. As the dose limiting factor increases, the tissue toxicity also increases with infiltration of lamina propria towards the submucosal lining, thickening of mucosa defined as fibrosis, dysplastic columnar cells and goblet cells in the maturation and absorption compartment, blenting of mucosal lining and villi erosion.
References
- Tekale.N.S. 2003. Idol immersion: a critical analysis of environmental impact on urban lakes and remedial measures. Souvenir/Abstracts PP:61-63. UGC sponsored National Conference on Urban lakes – Environmental status economics & management options, Hyderabad, India.
- Central Pollution Control Board (CPCB)., 2001-02. Environment Research, Chapter 7, Annual report.
- Sparling, D. W., T. P. Lowe, and P. G. C. Campbell. 1997. In Robert A. Yokel and Mari S. Golub, editors. Research issues in aluminum toxicity. Taylor & Francis, Washington, C. xi, 256; Pages 47-68.
- Barsiene J., 1995 Chromosome Set Changes in Molluscs from Highly Polluted Habitats, Genetics and Evolution of Aquatic Organisms, A. R. Beaumont (Ed.), Great Britain, Cambridge University Press, 102p.
- Kligerman, A. D. and Bloom, S. E., 1976 Sister Chromatid Differentiation and Exchanges in Adult Mudminnow (Umbra limi) after in vivo Exposure to 5-Bromodeoxyuridine, Chromosome, 56: 101-109.
- Zhang L., Rothman N., Wang Y., Hayes R.B., Li, G, Dosemeci M., Yin S., Kolachana P., Titenko-Holland N. and Smith M.T. 1955-1961, 1998. Increased aneusomy and Long Arm Deletion of Chromosomes 5 and 7 in the Lymphocytes of Chinese Workers Exposed to Benzene, Carcinogenesis, 19
- ICPEMC, 1988. Testing for Mutagens and Carcinogens; the Role of Short-Term Genotoxicity Assays, Mutat. Res., 205: 3-12.
- feiffer P., Goedecke W. and Obe G., Mechanisms of DNA Double-Strand Break Repair and Their Potential to Induce Chromosomal Aberrations, Mutagenesis, 15 (4): 289-302, 1999.
- Banerjee TK, Paul VI. 1993. Estimation of acute toxicity of ammonium sulphate to the fresh-water catfish, Heteropneustes fossilis. II. A histopathological analysis of the epidermis. Department of Zoology, Banaras Hindu University, Varanas, India. Biomed Environ Sci. Mar; 6(1):45-58.







