Manuscript accepted on :May 11, 2009
Published online on: --
Plagiarism Check: Yes
Dwarika Prasad¹ and S. P. Sati²
¹Department of Chemistry LPU, Punjab India.
²Department of Chemistry, Govt. P.G. College Gopeshwar Chamoli - 246 401 India.
Abstract
From bark of Scutellaria scandens a new a-D-Glycoside was isolated and characterized as 4-methoxycarbonyl-3-methyl phenyl-6’-O-4”-methoxy sinapoyl-á-D-glucopyranoside by FAB-mass and 1H and 13C NMR studies & antimicrobial activities of its extract.
Keywords
Scutellaria scandance; Lamicaeae; glycoside; 4-methoxycarbonyl-3-methyl
Download this article as:| Copy the following to cite this article: Prasad D, Sati S. P. A New Glycoside from Scutellaria Scandance Bark. Biomed Pharmacol J 2009;2(1) |
| Copy the following to cite this URL: Prasad D, Sati S. P. A New Glycoside from Scutellaria Scandance Bark. Biomed Pharmacol J. 2009;2(1). Available from: http://biomedpharmajournal.org/?p=695 |
Introduction
Scutellaria scandens belongs to the family Lamiaceae, is perennial erect shrub widely distributed in North Western Himalayas in India. Scutellaria species have been used as antimicrobial medicine for a variety of purposes. (1).From leaves of S. scandans. Pinosylvin -3-O-b-D glucopyranoside and 3,5-dihydroxy tras-stilbene-2-carboxylic acid, 2,4 dihydroxy-phenyl ethyl-6-O-sinapoly-b-D-glycopyranoside and 4-methoxy carbonyl methyl phenyl 6-sinapoly (2,3,) The chemical examination of Scutellaria species (4) has been reviewed. The present study has led to the isolation of a new α-D-Glycoside from an ethanolic extract of the bark of Scutellaria scandens. The structure of compound has been elucidated through extensive FAB-mass, 1H and 13C NMR studies.
Material and Methods
The bark of Scutellaria scandane were collected from Ganglogaun, Dist. Chamoli,Garhwal,Uttrakhand and identifed from Department of Botany P.G. College. Gopeshwar where Vaucher specimen was deposited. The air dried barks of plant (3kg) was exhaustively extracted with 90% aqueous ethanol for 72 hours. The ethanolic extract was chromatographed over silica-gel on elution of coloum with Methanol-Chloroform (31:69) afforded compound purified by crystallization from methanol.
Data
Colourless Crystalline Solid., FAB-m/z 549 (M+H)+, 1H-NMR (400 MHz –DMSO) (glycone –hexose) δ 3.80(s) , 4.75(d J=5.2Hz)), 4.85(t ,J=5.2 Hz), 4.90 (t, J=4.8 Hz) 4.25 (t, J=6.8Hz), 4.00 (dd, J=4.4,4.0 Hz), 4.60 (s), 4.45 (s), 4.50 (s). (aglycone) δ 5.15 (s), 5.60 (s), 5.15 (s), 3.1 (s), 1.2 (s). (sinapic acid) δ 5.3(s), 6.6 (d J=6.8 Hz), 6.25 (d, J=4.4 Hz), 3.6(s), 3.4(s). 13C-NMR (75 MHz, DMSO) (glycone-hexose) δ 100.4(C1’), 73.2(C2’), 76.4(C3’), 69.8(C4’), 73.8(C5’), 63.8(C6’) (aglycone) δ156.2(C1), 116.2(C2), 135.3(C3), 128.6(C4), 130.3(C5), 116.2(C6), 171.25(C7), 51.6(C8), 21.3(C9), (sinapic acid) δ 124.4(C1”), 106.2(C2”), 148.2(C 3”), 149.0(C4”), 148(C5”), 116.2(C6”), 145.5(C7”), 114.7(C8”), 166.5(C9”), 57.1(C10”), 56.5(C11”).
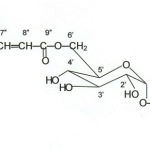 |
Scheme 1:
|
Result and Discussion
It was crystallized from methanol as colourless amorphous solid. It correspond the molecular ion peaks at m/z 549 [M+H]+, their by indicating 548 amu. is the molecular weight of compound, other fragment ions was observed at m/z 535, 429, 397,391, 369, 307 etc. in the positive ion FAB-MS. Elemental analysis of compound corresponded to the molecular formulae C27 H32 O12. The 1H-NMR spectrum of compound showed characteristic signals for anomeric proton (H-1′) at δ 3.8 (s) indicate the presence of α linkage. Acidic hydrolysis (7% MeOH-HCl, 10ml, 60 to 800C, 8hr) furnished aglycone (4-methoxycarbonyl-3-methyl phenol) identified by comparing its spectral data with that of literature (2) and D-glucose by co-TLC.
1H-NMR spectrum of the compound showed singlet at δ 5.3 for tetra-substituted benzene two sharp singlet at δ 3.6 and 3.4 for two methoxy groups respectively. The signals for two α-β-methine was observed at δ 6.6 (d, J=6.8 Hz) and δ 6.25 (d, J=4.4 Hz) for sinapic acid (6′-O-4”-methoxy-sinapoyl). Two singlet at δ 5.6 and 5.15 for trisubstituted benzene and sharp singlet at δ 3.1 and 1.2 for one methoxy of ester group and one methyl group respectively assigned for aglycone (4-methoxy carbonyl-3-methyl-phenyl). For sugar moiety, six peaks were observed at δ 3.8 (s), 4.75 (d, 4.4Hz). 4.85(t, 5.2 Hz) 4.9 (t, 4.8Hz) 4.0(dd, 7.2 & 10.4 Hz) and three singlet beak at δ 4.6(s), 4.45(s), 4.50(s) for hydroxy groups (2).
13C-NMR spectrum of the compound showed chemical shifts of the carbon signals at δ 100.4 (C-1′), 73.2 (C-2′), 76.4(C-3′), 69.8(C-4′), 73.8(C-5′), 63.3 (C-6′) which corresponding with analogous data of the glucose (2). The signals for aglycone part δ 156.2 (C-1) aromatic carbons, 116.2, 135.3, 128.6, 130.3, 116.2 for C-2, 3, 4, 5, 6 respectively. The signals at δ 171.25 (C-7) for–CO-O group δ 51.6 for methoxy carbon, δ 21.3 for methyl group. The signals for sinapic acid at δ 124.4 (C-1”), 106.2 (C-2”), 148.2 (C-3”), 149.2(C-4”), 148.0 (C-5”), 116.2 (C-6”) were reported for aromatic carbons and the signal for -CH=CH- were reported at δ 145.5 and 114.7 and δ 166.5 (C-9” -CO-O). Two signals were observed at for d 57.1 (C-10”) and 56.5 (C-11”) for methoxy groups. These data correlate with the reported data of sinapic acid (4).
Antibacterial activities
The ethanotic extract of Scutellaria scandans moderately active against two bacterial cultures as klebsiella pneumniae and Mycobacterium smegmatis by use Agar well diffusion method (5).
Acknowledgement
A authors express their thanks to Principal, Govt. P.G. College Gopeshwar Chamoli, for availing lab facility and SAIF, CDRI, Locknow for recording spectra, and SBSPG institute of Biomedical Dehradun for antibacterial activities.
Reference
- Gaur; R.D. “Flora of District Garhwal” Trans Media, Srinagar Garhwal 1999.
- Miyaichi –Y Imoto-Y., Kizu-H., Shoyakugaku Zasshi 1988, 42:3, 204.
- Ishiura-A.,Kizu-H., Tomimori-T., Shoyakugaku Zasshi 1993, 47:3, 287.
- Malikov V.M. Yuldashev M.P. Yuldashev, Khiin Prir Soedin, 2002, 5, 385.
- Cony, C.H., Lyne, M.P. and Grange, M.J. (1995) Microbiological Methods, 7th ed, Butter worth-Heinemann. Ltd, Great Britain







