V. J. Kaila, D. J. Desai and K .D. Patel*
Chemistry Department, V. P. and R. P. T. P. Science College, Vallabh Vidyanagar(India).
Abstract
A series of 2- substituted phenyl-3-(phenyl-(substituted amino) methyl amino)-5-(3',4',5'- trimethoxy benzylidene)-3,5-dihydro-imidazol-4-one derivatives were synthesized by the condensation of 2- substituted phenyl-4- (3',4',5'- trimethoxy benzylidene)- 4H-oxazol-5-one and Phenyl hydrazine in Pyridine, which was carried out by Mannich reaction in presence of formaldehyde and different secondary amines to afford title compounds. The synthesized compounds have been characterized on the basis of elemental analysis and spectral studies like IR, 1H-NMR, etc. Further they were assayed for their bactericidal activity against E.Coli, B.Subtilis bacterial species and A.niger fungal microorganism.
Keywords
Oxazolone; Imidazolone; Bactericidal activity; IR; NMR
Download this article as:| Copy the following to cite this article: Kaila V. J, Desai D. J, K .D. Patel K. D. Synthesis, Characterization and Bactericidal Activity of some Newly Nitrogen Containing Heterocyclic Moiety. Biomed. Pharmacol. J.2008;1(2) |
| Copy the following to cite this URL: Kaila V. J, Desai D. J, K .D. Patel K. D. Synthesis, Characterization and Bactericidal Activity of some Newly Nitrogen Containing Heterocyclic Moiety. Biomed. Pharmacol. J.2008;1(2). Available from: http://biomedpharmajournal.org/?p=454 |
Introduction
The heterocyclic chemistry in which same N-containing heterocyclic moiety have extra ordinary bactericidal activity. Imidazolone is one of the most important N-containing heterocyclic moieties, which has overlapping properties of both pyrrole and pyridine.
Among a wide variety of nitrogen heterocycles that have been explored for developing pharmaceutically important moiety. N-containing heterocyclic moiety oxazolone [1] and Imidazolone [2] have played an important role in medicinal chemistry, dyes, agricultural and many other fields.
Recently the chemistry of oxazolone has received important attraction due to their uses as intermediate for the synthesis of some heterocyclic synthesis [3]. Imidazolon-5-one have been found to possess potent CNS depressant [4], anticonvulsant [5], anti-inflammatory [6], sedative and hypnotic [7] and also trisubstituted imidazol-5-one have been reported to possess mono-amino-oxidase inhibitory activity [8].
A chemical substance produced by chemical synthesis, which inhibited the growth of organisms and hence act as antimicrobial agents. These chemical substances interfere the life cycle of organisms with specific processes that are essential for growth and/or division of the cell and many of them are widely used for chemotherapy. Most of the pathogenic bacteria are highly sensitive and susceptible to a new antibiotic or chemical, and thus microorganisms acquire chemical substance/drug resistance. In view of the advantage offered by applications of Mannich bases in the field of medicine and biology [9]. Bactericidal activity of various imidazolone derivatives were reported by researches, in view of this observation, we report here the synthesis of some novel heterocyclic N-Mannich bases of imidazol-5-one and evaluation of their bactericidal activity against bacterial and fungal microorganisms.
Hence, it was thought interesting to undertake the synthesis, characterization and bactericidal activity of tri substituted imidazol-5-one moiety. The whole work is represented in Scheme-I.
Experimental
Materials
All the chemicals used were of laboratorary grade and were further purified by recrystallization and redistilled before used.
Synthesis of 2-substituted phenyl – 4 – (3′, 4′, 5′-trimethoxy benzylidene) – 4H-oxazol – 5 – one
This was prepared by the well known Erlenmeyer- Plochl Azalactone synthesis method [10]. It is of bright yellow colour compound having M.P. 135°C and yield is 65 %. The structure of oxazolone compound [II] is shown in Scheme-I and was confirmed by an elemental analysis and spectral studies. Molecular formula, C19H17NO5 ( R= H); Anal. Found: C, 67.25%; H, 5.01%; N, 4.12%.
Found: C, 67.14%; H, 4.93%; N, 4.09%; IR (KBr): 1680 cm-1(-C=O), 1610 cm-1(-C=C- Phenyl ring vibration), 1120 cm-1 (-C-O-C-). 1H NMR: 6.5- 8.0 δ (m, 8H, Ar-H and Ar-CH=C- merged), 3.7 δ (S, 3H, Ar- OCH3).
Synthesis of 2- substituted phenyl-3-(phenyl amino)-5-(3′, 4′, 5′- trimethoxy benzylidene)-3, 5-dihydro-imidazol-4-one
A mixture of oxazolone (3.39 gm, 0.01mole) and phenyl hydrazine (1.62 gm, 0.015 mole) in dry pyridine was heated under reflux for 10 hrs under anhydrous condition. The excess pyridine is distilled off and then reaction mass is cooled and subsequently the reaction mixture was poured into ice-cold water containing conc. HCl. A solid started to separate out was allowed to settle down for sometimes. It was filtered off and wash sucessessively with water, dried and recrystallized from ethanol to give compound [IV]. M.P. is 218°C and Yield is 60 %.
The compound [IV] is shown in scheme-I and was confirmed by an elemental analysis and spectral studies. Molecular formula, C26H23N3O4 (R= H); Anal. Found: C, 70.58%; H, 5.42%; N, 9.50%. Found: C, 70.48%; H, 5.35%; N, 9.48%; IR (KBr): 1720 cm-1 (-C=O), 1120 cm-1(-C=C- Phenyl ring vibration), 1630 cm-1 (-C=N-), 1595 (S) cm-1 (-CH=C-), 1315 (W) cm-1(-C-N- Stretching), 300-3000 cm-1 (-N-H- Stretching). 1H NMR: 6.6- 8.2 δ (m, 13H, Ar-H and Ar-CH= C-); 8.9 δ (S, 1H, Ar-NH- N-), 3.81 δ (S, 3H, Ar- OCH3).
Synthesis of 2-substituted phenyl-3-[phenyl-(substituted amino)-1-ylmethyl-amino]- 5-(3′, 4′, 5′- trimethoxy benzylidene)-3,5-dihydro-imidazol-4-one
A mixture of compound [IV] (0.01 mole) and formaldehyde (0.02 mole) was refluxed in methanol (25 ml) for 1hr. A secondary amine (0.01mole) was added to the reaction mixture and then refluxed for 3 hrs. Methanol was distilled off and the product was crystallized from suitable solvent to give compound [V].
Similarly other compounds of this series were obtained by above method. 14 compounds have been prepared which are listed in Table-1.
The synthetic protocol for the synthesis of N-Mannich bases in general is furnished in the.
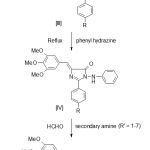 |
Scheme 1: Synthetic protocol of N-Mannich bases.
|
Measurments
C, H, and N content of the entire sample were estimated by Perkin-Elmer 2400 Series II, C, H, N, and S Elemental Analyzer, Italy. The IR spectra of the entire sample were scanned in KBr pellets on a NICOLET- 400 D FTIR spectrophotometer. The 1H-NMR spectral studies were carried out on 90-MHz FT-NMR instrument in CDCl3 as a solvent. Melting points were uncorrected and determined in open capillary. Purity of the compounds was checked by TLC on silica gel and was purified using column chromatography.
Table 1: Physical and analytical data of compounds.
| Compd.Nos. | R΄
|
Molecular Formula | M.P.*
°C |
%Yield | Elemental Composition | |||||
| % C | % H | % N | ||||||||
| Found | Calcd. | Found | Calcd. | Found | Calcd. | |||||
|
1 |
( R= H )
MPPI |
C31H34N4O4 |
143 |
62 |
70.62 |
70.70 |
6.36 |
6.42 |
10.60 |
10.64 |
| 2 | MPMO | C30H32N4O5 | 194 | 73 | 68.10 | 68.17 | 6.00 | 6.04 | 10.58 | 10.60 |
| 3 | MPNMP | C31H35N5O4 | 160 | 76 | 68.66 | 68.74 | 6.45 | 6.51 | 12.92 | 12.93 |
| 4 | MPPY | C30H32N4O4 | 127 | 68 | 70.20 | 70.29 | 6.24 | 6.29 | 10.90 | 10.93 |
| 5 | MPNEA | C34H34N4O4 | 189 | 67 | 72.49 | 72.58 | 6.05 | 6.09 | 09.92 | 09.96 |
| 6 | MPEI | C28H28N4O4 | 102 | 70 | 69.33 | 69.41 | 5.78 | 5.82 | 11.54 | 11.56 |
| 7 | MPIN | C34H30N4O4 | 183 | 72 | 73.03 | 73.10 | 5.35 | 5.41 | 10.00 | 10.03 |
| ( R= Cl ) | ||||||||||
| 1 | MPPI | C31H33N4O4Cl | 108 | 68 | 66.28 | 66.36 | 5.89 | 5.93 | 09.98 | 09.99 |
| 2 | MPMO | C30H31N4O5Cl | 150 | 75 | 63.92 | 64.00 | 5.49 | 5.55 | 09.93 | 09.95 |
| 3 | MPNMP | C31H34N5O4Cl | 179 | 72 | 64.57 | 64.63 | 5.90 | 5.95 | 12.12 | 12.16 |
| 4 | MPPY | C30H31N4O4Cl | 135 | 70 | 65.81 | 65.87 | 5.66 | 5.71 | 10.21 | 10.24 |
| 5 | MPNEA | C34H33N4O4Cl | 202 | 65 | 68.29 | 68.39 | 5.50 | 5.57 | 09.34 | 09.38 |
| 6 | MPEI | C28H27N4O4Cl | 90 | 73 | 64.72 | 64.80 | 5.18 | 5.24 | 10.75 | 10.80 |
| 7 | MPIN | C34H29N4O4Cl | 198 | 69 | 68.80 | 68.86 | 4.89 | 4.93 | 09.41 | 09.45 |
*uncorrected
Spectral data of N- Mannich bases
IR (ν, cm-1 )
1710- 1765 ( -C = O stretching of imidazolone),
1635- 1685 (-C = N- stretching), 1600- 1645 (-C = C streching),
1220- 1265 (-C-O- stretching of Ar-OCH3), 700- 750 (-C- Cl streching),
2830- 2945 (-CH stretching of methylene bridge),
1440- 1470 & 1350- 1380 (-CH streching of alkane),
3000- 3100 (-CH stretching of benzene ring).
1H NMR (δ, ppm)
(R= H)
MPPI 6- 8.0 δ (13H, m, Ar-H and Ar–CH=C- merged), 4.15 δ (2H, s, CH2 of
methylene bridge), 2.35 δ (2H, t, CH2 adjacent to N of piperidine ring),
1.45 δ (4H, m, CH2 at 3 and 4-position of piperidine ring), 3.98 δ (3H, s, Ar-OCH3).
MPMO 85- 7.90 δ (13H, m, Ar- H and Ar–CH=C- merged), 4.20 δ (2H, s, CH2 of
methylene bridge), 2.45 δ (2H, t, CH2 adjacent to N of morpholine ring),
3.54 δ (2H, t, CH2 adjacent to O of morpholine ring), 3.98 δ (3H, s, Ar-OCH3).
MPNMP 65- 7.95 δ (13H, m, Ar- H and Ar–CH=C- merged), 4.14 δ (2H, s, CH2 of
methylene bridge), 2.35 δ (2H, s, CH2 adjacent to N of N-methyl piperazine ring), 2.20 δ (3H, s, CH3 of N-methyl piperazine ring), 3.98 δ (3H, s, Ar-OCH3).
MPPY 6- 8.03 δ (13H, m, Ar- H and Ar–CH=C- merged), 4.18 δ (2H, s, CH2 of
methylene bridge), 2.45 δ (2H, t, CH2 adjacent to N of pyrrolidine ring),
1.52 δ (2H, t, CH2 at 3-position of pyrrolidine ring), 3.98 δ (3H, s, Ar-OCH3).
MPNEA 8- 8.15 δ (18H, m, Ar- H and Ar–CH=C- merged), 4.72 δ (2H, s, CH2 of
methylene bridge), 3.46 δ (2H, q , CH2 of –N-CH2CH3 of N- ethyl aniline),
1.07 δ (3H, t, CH3 of –N-CH2CH3 of N- ethyl aniline), 3.98 δ (3H, s, Ar-OCH3).
MPEI 7- 8.00 δ (13H, m, Ar- H and Ar–CH=C- merged), 4.27 δ (2H, s, CH2 of
methylene bridge), 1.82 δ (2H, t, CH2 of aziridine ring), 3.98 δ (3H, s, Ar-OCH3).
MPIN 65- 8.18 δ (19H, m, Ar- H and Ar–CH=C- merged), 4.92 δ (2H, s, CH2 of
methylene bridge), 3.98 δ (3H, s, Ar-OCH3).
(R= Cl )
MPPI 7- 7.93 δ (12 m, Ar- H and Ar–CH=C- merged), 4.07 δ (2H, s, CH2 of
methylene bridge), 2.20 δ (2H, t, CH2 adjacent to N of piperidine ring),
1.38 δ (4H, m, at 3 and 4-position CH2 of piperidine ring), 3.94 δ (3H, s, Ar-OCH3).
MPMO 6-8.0 δ (12H, m, Ar- H and Ar–CH=C- merged ), 4.12 δ (2H, s, CH2 of
methylene bridge), 2.35 δ (2H, t, CH2 adjacent to N of morpholine ring),
3.45 δ (2H, t, CH2 adjacent to O of morpholine ring), 3.94 δ (3H, s, Ar-OCH3).
MPNMP 6- 8.08 δ (12H, m, Ar- H and Ar–CH=C- merged), 4.02 δ (2H, s, CH2 of
methylene bridge), 2.27 δ (2H, s, CH2 adjacent to N of N-methyl piperazine ring),
2.13 δ (3H, s, CH3 of N-methyl piperazine ring), 3.94 δ (3H, s, Ar-OCH3).
MPPY 7-7.9 δ (12H, m, Ar- H and Ar–CH=C- merged ), 4.11 δ (2H, s, CH2 of
methylene bridge), 2.40 δ (2H, t, CH2 adjacent to N of pyrrolidine ring),
1.42 δ (2H, t, CH2 at 3-position of pyrrolidine ring), 3.94 δ (3H, s, Ar-OCH3).
MPNEA 7- 8.19 δ (17H, m, Ar- H and Ar–CH=C- merged),4.60 δ (2H, s, CH2 of
methylene bridge), 3.30 δ (2H, q , CH2 of –N-CH2CH3 of N- ethyl aniline),
1.00 δ (3H, t, CH3 of –N-CH2CH3 of N- ethyl aniline), 3.94 δ (3H, s, Ar-OCH3).
MPEI 77- 8.00 δ (12H, m, Ar- H and Ar–CH=C- merged), 4.14 δ (2H, s, CH2 of
methylene bridge), 1.9 δ (2H, t, CH2 of aziridine ring),
3.94 δ (3H, s, Ar-OCH3).
MPIN 7- 8.11 δ (18H, m, Ar- H and Ar–CH=C- merged), 4.80 δ (2H, s, CH2 of
methylene bridge), 3.94 δ (3H, s, Ar-OCH3).
Bactericidal Activity
Bactericidal study of N-Mannich bases of imidazol-5-one
The newly synthesized N-Mannich bases of imidazol-5-one were screened for their bactericidal activity by using different bacterial and fungal microorganisms. The test was performed by using the agar cup borer method with some modifications using Streptomycin and Imidazole as standard for bacterial and fungal culture respectively as shown in Table 2.
Table 2 : Bactericidal activity of compounds
| N-Mannich bases | Diameter of Zone of inhibition (in mm)
__________________________________________________________ E.coli B.subtilis A.niger |
||
| ( R= H ) | |||
| MPPI | 5 | 7 | 11 |
| MPMO | 18 | 19 | 16 |
| MPNMP | 5 | 6 | 7 |
| MPPY | 11 | 7 | 12 |
| MPNEA | 4 | 7 | 4 |
| MPEI | – | 6 | – |
| MPIN | 5 | 10 | 7 |
| ( R= Cl) | |||
| MPPI | 12 | 8 | 13 |
| MPMO | 20 | 20 | 18 |
| MPNMP | 9 | 8 | 8 |
| MPPY | 12 | 8 | 14 |
| MPNEA | 5 | 7 | 6 |
| MPEI | 3 | 9 | 5 |
| MPIN | 6 | 12 | 9 |
| STANDARD DRUGS
Streptomycin Imidazole
|
26 – |
24 – |
– 22 |
Antimicrobial assay
Agar cup borer method [11] was used for the evaluation of antimicrobial agents. A test tube containing sterile melted top agar (2 %) previously cooled to 50o C and with 0.2 ml suspension of the test culture, mixed thoroughly and poured in the Petri dish containing sterile base agar medium (Nutrient agar) and allowed it to solidify. The cup borer was sterilized by dipping into absolute ethanol and flaming it and then allowed to cool it down, with the help of sterile cup-borer; three cups in the agar-plate were marked and were injected with 0.1 ml of test solution, 0.1 ml of standard drug streptomycin in DMSO (Dimethyl sulfoxide) solvent and 0.1 ml of DMSO solvent respectively. Then the plates were allowed to diffuse for 20 min in refrigerator at 4-5°C. The plates were incubated in upright position at 37°C for 24 hrs and on the next day the zone of inhibition of surrounding each cup was observed.
Result and Discussion
The N-Mannich bases vary considerably in their range of effectiveness. Some are effective against a limited variety of microorganisms while some are broad spectrum. When chemical substance is added in agar cup, the radial diffusion through the agar produces a concentration gradient. Test organism is inhibited at the minimum inhibitory concentration, giving rise to a clear zone of inhibition.
Of these different N-Mannich bases, MPMO were found to have good activity against E.coli and B.subtilis, while MPPI, MPNMP and MPPY were found to be moderately active against E.coli. MPPI, MPPY and MPIN were found to be moderately active against B.subtilis and the other compounds had less or negligible activity against bacterial species respectively. MPMO were found to be highly active against A.niger. MPPI and MPPY were found as moderately active against A.niger as a fungal species while other compounds had less or no activity.
Conclusion
Bactericidal activity is increase when chloro group present on phenyl ring at 4-position. Morpholine containing compound also shows higher activity compared to other compounds. It was concluded that the synthetic compounds were found to have good application against the different bacterial as well as fungal species and provide significant role for drug designing and other pharmaceuticals approaches. Determination of effectiveness of chemical substance against a specific pathogen is essential to proper therapy.
Acknowledgment
The authors are thankful to Chairman, Charutar Vidya Mandal, Vallabh Vidyanagar for providing necessary research facilities.
References
- Vogel A.L., A text Book of Practical Organic Chemistry, ELBS-Longmans, London, pp.999, (1971).
- Solankee Anjani, Kapadia Kishor, Patel Jayesh and Thakor Indrajit., Asian J. Chem., 14,699 (2002).
- EI-Magharaby A., Abou EI-Ela A., Khalafalla A.K. and EI-Shawi E., J. Indian Chem. Soc., 62,676 (1985).
- Chadha T.C., Mahal H.S. and Venkataraman, J. Chem. Soc., 1459 (1933).
- Pandya K.C., Kurion R.N. and Surange V.R., J. Indian Chem. Soc., 11,823 (1934).
- Swarup S., Saxena V.K. and Chaudhary S.R., India J. Pharm. Sci., 51,124 (1989).
- Goldberg M.W. and Lehr H.H., Chem Abstr., 47,6987d (1953).
- Dwivedi C., Halbison R.D., Ali B. and Parmar S.S., J. Pharm. Sci., 63,1124 (1974).
- Feldmann J.R. and Wagner E.C., J.Org. Chem., 7, 31 (1942).
- V.K.Ahluwalia and Renu Aggrawal,” Comprehensive Practical Organic Chemistry-Quantitative Analysis”, pp.82, 2004.
- Verma R.S. and Imam S.A., Indian J. Microbiol., 13, 45 (1993).







