Eswar Kumar¹, Sk. Mastan2*, N. Sreekanth and G. Chaitanya
¹Pharmacology Division, University College of Pharmaceutical Sciences, Andhra University, Visakhapatnam (India).
2Department of Pharmacology, Roland Institute of Pharmaceutical Sciences, Ambapua, Berhampur (GM)(India).
Corresponding Author E-mail:shkmastan@gmail.com
Abstract
Since drugs from oriental medicine are used along with allopathic drug therapy in highly populated countries, such as India and China, and since optimal blood sugar control is needed in diabetes, an aromatic herb/spice that influences blood glucose was studied for its effect on tolbutamide response in normal and diabetic rats. In the present study the safety of the aromatic herb/spice–drug combination with respect to blood glucose in animal models was studied. Albino rats of either sex were divided into three groups of six each and were fasted for 18 h prior to the experiment with water ad libitum. Three groups received aqueous extract of Carum carvi fruits (CAE), tolbutamide 40 mg/kg body weight and CAE prior to the administration of tolbutamide 40 mg/kg body weight respectively in normal and diabetic rats. All the groups were administered orally. Blood samples were collected from the retro-orbital plexus at 0 (pre dose), 1, 2, 4, 6, 8 and 12 h after drug administration and were analyzed for blood glucose by glucose oxidase/peroxidase (GOD-POD) method. Diabetes was induced by alloxan monohydrate 100 mg/kg body weight administered by i.p route. Tolbutamide/CAE produced hypoglycemic activity in a dose dependent manner in normal and diabetic condition with peak activity at 6 h. In the presence of CAE, tolbutamide produced early on set of action and maintained for longer period compared to tolbutamide group. The study indicated that the combination can be used safely to obtain prolonged and sustained antidiabetic effect.
Keywords
Diabetes; Caraway; Alloxan monohydrate; Hypoglycemia; Antihyperglycemia
Download this article as:| Copy the following to cite this article: Kumar E, Mastan S, Sreekanth N, Chaitanya G. Influence of Aqueous Extract of Carum Carvi Fruits on Tolbutamide-Induced Hypoglycemia/Antihyperglycemia in Normal/alloxan-Induced Diabetic Rats. Biomed. Pharmacol. J.2008;1(2) |
| Copy the following to cite this URL: Kumar E, Mastan S, Sreekanth N, Chaitanya G. Influence of Aqueous Extract of Carum Carvi Fruits on Tolbutamide-Induced Hypoglycemia/Antihyperglycemia in Normal/alloxan-Induced Diabetic Rats. Biomed. Pharmacol. J.2008;1(2). Available from: http://biomedpharmajournal.org/?p=456 |
Introduction
Diabetes mellitus is a major metabolic disorder characterized by chronic hypoglycemia as a result of a relative or absolute lack of insulin or the actions of insulin [1]. It is estimated that in the year 2010 more than 200 million people world wide will have diabetes mellitus and 300 million people will subsequently have the disease in 2025 [2, 3]. Most of these cases will be type-2 diabetes, which is strongly associated with a sedentary life style and high calorie-nutrition and obesity [4]. Diabetic patients are shown to have increased oxidative stress and decreased antioxidant levels [5, 6].The major mode of control over diabetes can be achieved by diet and exercise, insulin replacement therapy and by the use of oral hypoglycemic agents [7]. Foods of high fiber content like fruits, vegetables, grains, beans and foods that contain less amount of fat, cholesterol, sugar and salt are useful in maintaining normal glucose levels in diabetic patients.
Carum carvi, which belongs to the family Umbelliferae is a biennial herb widely cultivated in West Asia, Europe and North America. The fruit of Carum carvi (caraway fruit) has an aromatic fresh taste and smell caused by an essential oil contained in ducts of the pericarp [8]. Caraway fruits have been reported to have hypolipidemic, hypoglycemic [9], free radical scavenging activities and useful to control postprandial rise of blood glucose particularly in diabetic condition [10]. Chemical analysis reveals that the plant contains proteins, essential amino acids, phosphorus, calcium, potassium, magnesium, sodium, petroselinic acid and polysaturated fatty acids (oleic acid and linoleic acid) [11]. The fruits contain several antioxidant constituents, such as monoterpenes (carvone, limonene), germacrene D and transdihydrocarvone, glucoside, quercetin, kaempferol [12]. Use of caraway fruits for the treatment of diabetes has been a common practice in countries such as India and China since ancient times. Moreover, caraway fruits are used in the preparation of curries etc in particularly in Indian/Chinese foods [10]. In India and China, people often use both herbs/vegetables/spices and drugs together. In such situation, the herb/spice may interact with the drug, thereby enhancing or reducing the effects of the drug. Literature evidence indicates that some herbs interact with oral hypoglycemic agents [13].
Insulin is the drug of choice in type-1 diabetes and sulfonylureas are the drug of choice in type-2 diabetes. Among sulfonylureas, Tolbutamide is the drug of choice for geriatrics because of its short duration of action and lower incidence of hypoglycemia in early hours of night.
In the present study, the influence of aqueous extract of Carum carvi fruits (CAE) on hypoglycemia and antihyperglycemia of tolbutamide in rats was studied to evaluate the safety of the combination with respect to blood glucose.
Material and Methods
Albino rats (175-200 g) were procured from Mahaveer Enterprises, Hyderabad, India were used in the study. They were maintained under standard laboratory conditions at ambient temperature of 25±2oC and 50±15% relative humidity with a 12-h light/12-h dark cycle. Rats were fed with a commercial pellet diet (Rayans Biotechnologies Pvt Ltd., Hyderabad) and water ad libitum. The animals were divided into 3 groups of 6 each. They were fasted for 18 h prior to the experiment (allowing access to water) and, during the experiment, food and water were withdrawn. The experiments were performed after prior approval of the study protocol by the institutional animal ethics committee of Roland Institute of Pharmaceutical Sciences, Berhampur, India. The study was conducted in accordance with the guidelines provided by Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
The aqueous extract of Carum carvi fruits (CAE) was obtained from M/s Laila Impex, Vijayawada as a gift sample and it was used throughout the study. Alloxan monohydrate was purchased from LOBA Chemie, Mumbai, India. Tolbutamide was supplied by Dr Reddy’s Laboratories Ltd, Hyderabad, India. Glucose kits (Span diagnostics) were purchased from the local pharmacy. All other chemicals used for this study were analytical grade.
Study in normal rats
Albino rats of either sex were divided in to three groups.
Group-1 : CAE (200 mg/kg body weight by oral administration)
Group-2 : Tolbutamide (40 mg/kg body weight by oral administration)
Group-3 : CAE prior to the administration of Tolbutamide
Study in diabetic rats
Albino rats of either sex were treated with alloxan monohydrate (100 mg/kg body weight i.p.). Alloxan monohydrate was dissolved in saline solution and was administered. Animals were treated with 10% dextrose orally to combat the early phase of hypoglycemia. Rats showing fasting blood glucose levels above 200 mg/dL were selected for the study. These rats were divided in to three groups.
Group-1 : CAE (200 mg/kg body weight by oral administration)
Group-2 : Tolbutamide (40 mg/kg body weight by oral administration)
Group-3 : CAE prior to the administration of Tolbutamide
Collection of blood samples
Blood samples were collected from the retro orbital plexus of each rat at 0 (pre dose), 1, 2, 4, 6, 8 and 12 h (after drug administration). Blood glucose levels were determined by using GOD-POD method [14].
Statistical analysis
Data were expressed as mean ± SEM. The significance of blood glucose reduction produced by CAE + tolbutamide compared with Tolbutamide was determined by applying student’s unpaired t-test. p values of <0.05 were considered to be statistically significant.
Reseults
Effect in normal rats
In normal rats, oral administration of CAE at the dose of 200 mg/kg body weight and 40 mg/kg body weight of tolbutamide produced 24.46% and 44.60% blood glucose reduction respectively at 6 h as peak effects. In the presence CAE 200 mg/kg body weight, the action of tolbutamide was early in onset and maintained for 8 h. The data was presented in table 1.
Table 1: Mean Percent Blood Glucose Reduction In Normal Rats.
| Time (hr) | CAE
(Group-1) |
Tolbutamide
(Group-2) |
CAE + Tolbutamide
(Group-3) |
| 0 | 0 | 0 | 0 |
| 1 | 04.08 ± 1.14 | 14.46 ± 1.20 | 16.25 ± 1.64 |
| 2 | 06.12 ± 1.26 | 20.44 ± 1.26 | 24.56 ± 1.80* |
| 4 | 16.52 ± 1.30 | 39.64 ± 1.32 | 50.64 ± 1.67* |
| 6 | 24.46 ± 1.46 | 44.60 ± 1.16 | 62.21 ± 1.46* |
| 8 | 12.84 ± 1.56 | 39.46 ± 1.42 | 48.46 ± 1.38* |
| 12 | 06.32 ± 1.32 | 28.46 ± 1.20 | 30.25 ± 1.64 |
CAE: Aqueous extract of Carum carvi fruits
All values as mean ± SEM
* Significance at p<0.05
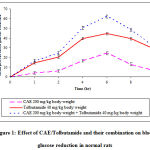 |
Figure 1: Effect of CAE/Tolbutamide and their combination on blood glucose reduction in normal rats.
|
Effect in diabetic rats
In diabetic rats, oral administration of CAE at the dose of 200 mg/kg body weight and 40 mg/kg body weight of tolbutamide produced 28.80% and 48.60% blood glucose reduction respectively at 6 h as peak effects. In the presence of CAE 200 mg/kg body weight, the tolbutamide produced antidiabetic activity at 1 h and was maintained for 12 h. The data was presented in table 2.
Table 2: Mean Percent Blood Glucose Reduction In Diabetic Rats.
| Time (hr) | CAE
(Group-1) |
Tolbutamide
(Group-2) |
CAE + Tolbutamide
(Group-3) |
| 0 | 0 | 0 | 0 |
| 1 | 3.04 ± 1.26 | 14.36 ± 1.20 | 18.64 ± 1.30* |
| 2 | 5.80 ± 1.38 | 20.32 ± 1.36 | 24.56 ± 1.46* |
| 4 | 14.60 ± 1.46 | 38.64 ± 1.40 | 48.64 ± 1.36* |
| 6 | 28.80 ± 1.24 | 48.60 ± 1.28 | 69.86 ± 1.52* |
| 8 | 24.62 ± 1.36 | 39.80 ± 1.38 | 54.62 ± 1.46* |
| 12 | 10.40 ± 1.48 | 30.26 ± 1.38 | 42.06 ± 1.48* |
CAE: Aqueous extract of Carum carvi fruits
All values as mean ± SEM
* Significance at p<0.05
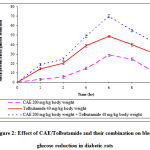 |
Figure 2: Effect of CAE/Tolbutamide and their combination on blood glucose reduction in diabetic rats.
|
Discussion
Drug-interaction studies are usually conducted in animal models to assess the safety of the combination, before they are conducted in humans. The normal rat model served to quickly identify the interaction, and the diabetic rat model served to validate the interaction in an actual-use condition of the drugs. The rat model was used for the pharmacodynamic-interaction study, since it is the most widely used species in drug metabolism and drug interaction studies.
The CAE and tolbutamide showed hypoglycemia/antihyperglycemia in normal/diabetic rats in dose-dependent manner. CAE and tolbutamide when administered alone produced the maximum effects at 6 h in normal and diabetic rats. Better reduction in blood glucose in diabetic condition indicating that Caraway fruits are more useful in stress (diabetes) than in normal condition. The exact mechanism to the effect of caraway on blood glucose has not been reported, it could be suggested that the antioxidant activity of caraway is responsible due to cumin and other flavanoids present in the fruits [15]. The fruits also contain other antioxidant constituents, such as monoterpenes (carvone, limonene), germacrene D and transdihydrocarvone, glucoside, quercetin, kaempferol [16]. Several lines of evidences suggest that increased oxidative stress occurs in diabetes mellitus and could have a role in the development of deterioration of peripheral insulin resistance. Alloxan has been observed to cause a massive reduction of the β-cells of the islets of Langerhans and induce hyperglycemia [17]. Moreover, alloxan was reported to produce diabetes by damaging pancreas by free radical related mechanisms [18]. Thus, it is logical to think that antioxidants can prevent precipitation of diabetes mellitus and also control hyperglycemia. Use of typical antioxidants alone/in combination may retard or even prevent the normal progression of diabetic complications.
In presence of CAE the onset of action of tolbutamide was early and maintained for longer duration compared to tolbutamide control. This effect was more significant in diabetic rats. Tolbutamide acts by stimulating insulin secretion [19] and also by increasing tissue up take of glucose [20]. The enhanced effect of tolbutamide in the presence of CAE might be due to antioxidant constituents of CAE. However, no convulsions were seen in rats, even at peak hours of activity in normal and diabetic condition. Hence, their combination need not be contraindicated.
The results from our study are clearly indicating that the combination of tolbutamide and aqueous extract of Carum carvi fruits (CAE) can be used safely with respect to blood glucose to obtain a prolonged and sustained antidiabetic effect. Further studies are needed to establish its long-term safety in animals and humans.
Acknowledgements
Authors are thankful to Prof. M.E. Bhanoji Rao, Principal, Roland Institute of Pharmaceutical Sciences for his kind support and encouragement.
References
- Kumar P.J. and Clark M., Textbook of Clinical Medicine, Saunders, London, 1099 (2002).
- Zimmet P., J. Intern. Med., 247, 301 (2000).
- King H., Aubert R. and Herman W., Diabet. Care., 21, 1414 (1998).
- Zimmet P., Shaw J. and Alberti K.G., diabet. Med., 20, 693 (2003).
- Jain S.K., Mcvie R., Jaramillo, J.J., Palmer M. and Smith T., J. Am. Coll. Nutr., 15, 458 (1996).
- Sardas S., Yimazz M., Oztak U., Cakir N. and Karakaya A.E., Mutation Res., 490, 123 (2001).
- Ivorra M.D., Paya M. and Villar A., J. Ethnopharmacol., 27, 243 (1989).
- Toxopeus H. and Bouwmeester H.J., Indus. Crops Prod., 1, 295 (1992).
- Modu S., Gohla K. and Umar I.A., Biokemistri, 7, 28 (1997).
- Sushruta K., Satyanarayana S., Srinivas N. and Rajasekhar J., Trop. J. Pharm. Res., 5(2), 613 (2006).
- Abdel A. and Attia M.R.S., Alexander Science Exchange, 4, 22 (1993).
- Iacobellis N.S., Cantore P.L., Capasso F. and Senatore F., J. Agric. Food Chem., 53, 57 (2005).
- Fetron C.W. and Avila J.R., Professionals Handbook of Complementary and Alternative Medicine, springhouse, Springhouse Corporation, PA, USA. (1999).
- Trinder P., Ann. Clin. Biochem., 6, 24 (1969).
- Kallio H., Kerrola K. and Alhonmaki P., J. Agr. Food. Chem., 42(11), 2478 (1994).
- Iacobellis N.S., Cantore P.L., Capasso F. and Senatore F., J. Agric. Food Chem., 53, 57 (2005).
- Goldner M. and Gomori G., Endocrinology, 33, 297 (1943).
- Heikkila R.E., Eur. J. Pharmacol., 44(2), 191 (1977).
- Vigneri R., Pezzino V. and Wang K.Y., J. Clin. Endocrinal Metab., 54, 95 (1982).
- Peifer M.A., Halter J.B., Beacd J.C. and Partel D.J., J. Clin. Endocrinal Metab., 53, 1256 (1981).







