M. Rama Rao1*, S. V. Raja Gopal2, I. Bhaskar Reddy, A. D. Naveen Kumar, A. Ramesh and T. Kartika
1Department of Biochemistry,College of Science, GITAM Univeristy, Visakhapatnam.
2Department of Biotechnology, College of Science, GITAM Univeristy, Visakhapatnam (India).
Corresponding Author E-mail:: ramaraomalla@rediffmail.com
Abstract
Holoptelea intigrifolia is traditionally used for the treatment of inflammatory disease, hemorrhoids. The role of free radicals has been proved in several diseases of inflammatory origin. To understand the pharmacological actions in inflammatory diseases, different types of radical scavenging and 51-lipoxygenase inhibitory activities of methanol extract of bark of H. intigrifolia have been investigated. Methanol extract exhibited significant radical scavenging activities with IC50 values of 105, 115, 265 and 105 mg/ml against 2, 2-diphenyl-1-picrylhydrazyl, super oxide, hydroxyl and nitric oxide radicals respectively. It also showed considerable inhibitory activity against 51-lipoxygenase with IC50 of 55.05 mg/ml. These results can contribute to understand the mechanism involved in the anti-inflammatory effects of the bark extract.
Keywords
Inflammatory disease; hemorrhoids; nitric oxide radical; super oxide radical
Download this article as:| Copy the following to cite this article: Rao M. R, Gopal S. V. R, Reddy I. B, Kumar A. D. N, Ramesh A, Kartika T. Effect of Holoptelea Intigrifolia Bark Extract on Radical Scavening and 5’-Lipoxygease Activities. Biomed. Pharmacol. J.2008;1(2) |
| Copy the following to cite this URL: Rao M. R, Gopal S. V. R, Reddy I. B, Kumar A. D. N, Ramesh A, Kartika T. Effect of Holoptelea Intigrifolia Bark Extract on Radical Scavening and 5’-Lipoxygease Activities. Biomed. Pharmacol. J.2008;1(2). Available from: http://biomedpharmajournal.org/?p=452 |
Introduction
Free radical-mediated oxidative damage has been implicated in the pathogenesis of number of inflammatory diseases. Treating these diseases with antioxidants is a rational approach and expected to have significant effect. Plants represent a vast untapped resource of bioactive molecules, including antioxidant and anti-inflammatory compounds. Many plant species have been investigated for the presence of antioxidants (Buyukokuroglu et al. 2001), (Shahidi & Wanasundara, 2002) still several other plant species have to be investigated for the presence of potential antioxidant and anti-inflammatory compounds.
Holoptelea intigrifolia is commonly known as Indian elm, belongs to the family Ulmaceae. The bark and leaves are bitter, astringent, thermogenic, anti-inflammatory, digestive, carminative, anthelmintic, and urinary astringent. They are useful in the treatment of inflammations, dyspepsia, helminthiasis, skin diseases, leprosy, diabetes and haemorrhoids (Vaidyaratnam, 1995). The bark is also used for the treatment of rheumatic swellings (Jayvir, 2002).
In the present study, methanol extracts of the bark of H. intigrifoliea are used for the determination of 2, 2-diphenyl-1-picrylhydrazyl, superoxide, hydroxyl, hydrogen peroxide and nitric oxide radical scavenging activities and 51-lipoxygenase inhibitory activity in order to assess the antioxidant and anti-inflammatory properties.
Materials and methods
TBA (thiobarbituric acid), DPPH (2, 2-diphenyl-1-picrylhydrazyl), ferrozine, TPTZ (2, 4, 6-tripyridyl-s-triazine), NBT (nitroblue tetrazolium), Naphthylenediamine dihydrochloride and Nordihydroguanaretic acid are obtained from Sigma Chemical Company, USA. All the chemicals used in the present study are of analytical grade and obtained from local suppliers.
Plant extract
The bark of H. intigrifolia is obtained from forest region of East Godavari district, Andhra Pradesh, India. The bark is authenticated by the department of Botany, Andhra University. The bark is thoroughly cleaned, shade dried and powdered in a mechanical grinder. The powder is extracted with 250ml of methanol using a Soxhlet extractor for 72 hrs. The extract is filtered using Whatman (No.1) filter paper and then concentrated in vacuum to dryness. Different concentrations of extracts are separately prepared by dissolving 10, 50,100,250,500 and 1000µg of dry residue in one methanol, mentioned as MHI and used to assay different radical scavenging activities and 51-lipoxygenase inhibitory activity. BHT and quercetin are used as positive controls for radical scavenging assays and Nordihydroguanaretic acid is used as positive control for lipoxygenase inhibition assay.
Total antioxidant power
The total antioxidant power is determined by the modified FRAP (Ferric chloride reducing ability of plasma) method of Benzie & Strain (1996). To 3.0ml of FRAP reagent (2.5ml of 0.3M acetate buffer, pH 3.6, 0.25ml of 10mM 2, 4, 6-tripyridyl-s-triazine (TPTZ) solution and 0.25ml of 20mM ferric chloride) 0.1ml of MHI or BHT or Quercetin at different concentrations is added and absorbance is measured at 595nm. Blank is set up with 3.0 ml of FRAP reagent and 0.1ml of methanol and proceed as per the test. The calibration curve was prepared using FeSO4 with concentrations ranging from 0-1mM. The results are expressed as Ascorbic acid Equivalent Antioxidant Capacity (AEAC) in terms of mM.
DPPH radical scavenging activity
DPPH scavenging activity is measured by the method of Koleva, Van Beek, Linssen, De Groot & Evstatieva, (2002). To 1.0 ml of an ethanolic solution of DPPH (0.3mM), 2.5 ml of MHI/ BHT/ Quercetin at different concentrations are added. For control, test sample is replaced by methanol. The contents are incubated at 37oC for 30 min and absorbance is measured at 517 nm using spectrophotometer. The percent inhibition of DPPH radical is calculated by the formula Ao–A x 100/ Ao. Where fore, Ao is Absorbance of control and A is Absorbance of test sample. IC50 values denote the concentration of sample, which is required to scavenge 50% of DPPH free radicals.
Super oxide radical scavenging activity
The super oxide scavenging activity is measured by Beauchamp & Fridovich method (1971) with some modifications. Superoxide anions are generated in a non-enzymatic hydroxyl amine (HA) – EDTA system and assayed by the reduction of nitroblue tetrazolium. The super oxide anion is generated in a reaction mixture containing 1.0 ml of sodium carbonate (125mM), 0.4ml NBT(25mM) and 0.2ml of EDTA (0.1mM) and 0.4ml of hydroxyl amine (0.1mM). The reaction is initiated by adding 0.5ml of different concentrations of MHI or BHT or Quercetin to the mixture. After 5 min of incubation at room temperature, the absorbance at 560 nm is measured in spectrophotometer. The control is simultaneously run without plant extract. The super oxide anion scavenging activity is calculated as percent inhibition of absorbance compared to the control.
Hydroxyl radical scavenging activity
The ability of the sample to inhibit hydroxyl radical mediated peroxidation is carried out according to method of Kunchandy & Rao (1990). The reaction mixture contained 0.1ml of different concentration of MHI or BHT or Quercetin, 0.5ml of 0.6mM deoxyribose in phosphate buffer (25mM, pH 7.4), 0.2ml of premixed 0.02mM ferrous ammonium sulfate and 0.02 mM EDTA (1:1 v/v) solution, 0.1ml of ascorbic acid (0.6mM) and 0.1ml of H2O2 (0.85mM), incubated for 15 min at 370C. After incubation, 1.5 ml of 2.8% cold TCA and 1.0ml of TBA are added. The concents are vortexed and heated in a water bath at 50oC for 15 min. The absorbance is determined at 532nm. Control is set up with out plant extract. The percentage of inhibition values are calculated from the absorbance of the control (Ao) and of the sample (A) using the formula, Ao–A x 100/ Ao.
Hydrogen peroxide scavenging activity
Hydrogen peroxide scavenging activity of the extract is estimated by the method of Zhang (2000). 1.0ml of 0.1mM H2O2 and 1.0ml of various concentrations of MHI or BHT or Quercetin are mixed, followed by 2 drops of 3% ammonium molybdate, 10ml of 2M H2SO4 and 0.7 ml of 1.8M KI. The mixed solution is titrated with 5.09mM Na2S2O3 until yellow color is disappeared. For control, all reagents are added except plant extract. Percentage of scavenging of hydrogen peroxide is calculated as percent inhibition.
Nitric oxide scavenging activity
Nitric oxide radical scavenging activity is determined according to the method reported by Garrat (1964). Sodium nitroprusside in aqueous solution at physiological pH spontaneously generates nitric oxide ions, which can be determined by the use of Griess Illosvoy reaction. 2.0 ml of 10mM sodium nitroprusside and 0.5ml phosphate buffer saline (pH 7.4) is mixed with 0.5ml of extract of various concentrations and the mixture is incubated at 25oC for 150min. From the incubation mixture 0.5ml solution is taken out and added to 1.0ml of sulfanilic acid reagent (33% in 20% glacial acetic acid) and incubated at room temperature for 5 min. Finally 1.0 ml of naphthylenediamine dihyrochloride (0.1%w/v) is mixed and again incubated at room temperature for 30 min and the absorbance at 540nm is measured with a spectrophotometer. For control, methanol is used in place of plant extract. The nitric oxide radical scavenging activity is calculated as percent inhibition.
Fe2+ chelating activity
The chelating activity of MHI or BHT or Qercetin for ferrous ions is measured according to the method of Dinis, Madeira & Almeidam (1994). To 0.5 ml of extract at different conetrations, 1.6ml of distilled water and 0.05ml of FeCl2 (2mM) are added. After 30 sec, 0.1 ml of ferrozine (5mM) is added. Ferrozine react with the divalent iron to form stable magenta complex soluble in water. After 10 min at room temperature, the absorbance of the Fe2+-Ferrozine complex is measured at 562nm. Control is run simultaneously with out plant extract. The chelating activity of the extracts for Fe2+ is calculated as percent chelating rate using formula, Ao–A x 100/ Ao
In vitro inhibition of lipid peroxidation
Lipid peroxidation induced by FeSO4-ascorbate system in sheep liver homogenate is estimated as thiobarbituric acid reacting substances (TBARS) by the method of Ohkawa, Ohishi & Yagi (1979) The reaction mixture contained 0.1ml of sheep liver homogenate (25%) in Tris-HCl buffer (20mM, pH 7.0; KCl (30mM); FeSO4 (NH4) SO4.7H2O (0.06 mM) and various concentrations MHI or BHT or Qercetin in a final volume of 0.5ml and incubated at 37oC for 1h. After the incubation, 0.4ml is removed and treated with 0.2ml sodium dodecyl sulphate (8.1%), 1.5ml thiobarbituric acid (TBA) (0.8%) and 1.5ml of trichloroacetic acid (20%). The total volume is made up to 4.0ml with distilled water and then kept in a water bath at 95oC for 1h. After cooling, 1.0ml of distilled water and 5.0ml of n-butanol and pyridine mixture (15:1) are added to the reaction mixture, shaken vigorously and centrifuged at 4000g for 10 min. The butanol pyridine layer is removed and its absorbance is measured at 532 nm. Control is also run in the same manner but plant extract is replaced with methanol. Inhibition of lipid peroxidation is determined by comparing the optical density (OD) of the test sample with that of the control.
51-lipoxygenase assay
Inhibition of the 51-lipoxygenase activity is determined using the method developed by Sircar, Shwender & Johnson (1983) and modified by Evans (1987). The standard assay mixture contained 0.01ml of plant extract or BHT or Qercetin dissolved in dimethyl sulfoxide, 2.95 ml of potassium phosphate buffer, pH 6.3 and 0.1ml of linolic acid. The reaction was initiated with the addition of 0.01ml of 51-lipoxygenase diluted with an equal of volume of potassium phosphate buffer and maintained at 4oC. The change in absorbance at 234nm is recorded for 10 min using spectrophotometer. DMS is used in place of plant extract for control. The percentage of inhibition is calculated by comparing with control (DMS). Nordihydroguanaretic acid (NDGA) is used as positive control.
Statistical analysis
All the experiments are performed thrice and results are the mean of five replicates. The statistical significance of a treatment effect is evaluated by student’s t-test and the values are expressed as mean ± SD. Probability limit is set at p<0.05.
Results and Discussion
Free radicals are inextricably linked to the inflammatory process. Many plant products exert antioxidant effect by quenching various free radicals and the singlet form of molecular oxygen. Various methods have been proposed to evaluate antioxidant characteristics and to explain antioxidant function of plant products. Of these, antioxidant activity, reducing power, metal chelation, and different types of free radical scavenging activities are most commonly used for the evaluation of the total antioxidant behavior of extracts (Elmastas et al. 2006).
The total antioxidant power of plant extract (MHI), synthetic antioxidant (BHT) and natural antioxidant (Quercetin) is determined and results are expressed in AEAC in term of mM. The MHI showed significant total antioxidant power with an AEAC value of 0.57mM, which is higher than synthetic antioxidant BHT (0.49mM), Quercetin (0.47mM) (fig.1).
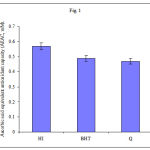 |
Figure 1: Values are means ± SD (n=5).
|
DPPH is a stable free radical that accepts an electron or hydrogen radical to become a stable diamagnetic molecule. Hence DPPH is usually used as substrate to evaluate the antioxidant activity (Elmastas et al. 2006). The strength of the scavenging activity of methanol extract and standards on DPPH radical followed the order of MHI>BHT>Q with percentage of inhibitions of 93.70, 84.14 and 73.50 at 1mg/ml respectively. These results indicated that MHI has a significant effect on scavenging free radicals. Free radical scavenging activity also increased with increasing concentration (Table 1). Based on the data obtained from this study, MHI is free radical scavenger as well as primary antioxidant that react with free radicals, which may limit free radical damage occurring in the human body.
Table 1:
| Concentration (µg/ml) | Percentage of inhibition by | ||
| H. intigrifolia | Butylated hydorxy toluene | Quercetin | |
| 10 | 37.14±0.18 | 32.18±0.31 | 26.08±0.31 |
| 50 | 42.15±0.16 | 42.21±0.22 | 32.15±0.18 |
| 100 | 49.18±0.20 | 49.01±0.41 | 37.18±0.31 |
| 250 | 59.41±0.27 | 56.12±0.20 | 48.31±0.21 |
| 500 | 65.44±0.21 | 61.20±0.31 | 59.21±0.22 |
| 750 | 76.41±0.22 | 72.65±0.30 | 65.26±0.31 |
| 1000 | 93.70±0.32 | 84.14±0.12 | 73.50±0.31 |
| IC50 | 105 | 210 | 325 |
Values are means ± SD (n=5)
DPPH radical scavenging activity of methanol extracts of H. intigrifolia
Superoxide anion is one of the most representative free radicals. In biochemical systems, superoxide radicals can be converted to hydrogen peroxide by the action of dismutase and the H2O2 can subsequently generate extremely reactive hydroxy radicals in the presence of certain transition metal ions. Hydroxy radicals can attack DNA molecules to cause strand scission. Superoxide anion scavenging activity of MHI is investigated and compared to BHT and Quercetin (table 2). The IC50 of 115 mg/ml is observed for the plant extract is low compared to BHT (121mg/ml) and Quercetin (249mg/ml).
Table 2:
| Concentration (µg) | Percentage of inhibition by | ||
| H. intigrifolia | Butylated hydorxy toluene | Quercetin | |
| 10 | 12.08±0.02 | 11.02±0.02 | 09.12±0.03 |
| 50 | 24.23±0.04 | 25.24±0.03 | 21.41±0.04 |
| 100 | 42.32±0.03 | 39.42±0.02 | 36.12±0.03 |
| 250 | 65.49±0.04 | 62.12±0.03 | 50.52±0.03 |
| 500 | 78.62±0.03 | 69.56±0.04 | 63.06±0.04 |
| 750 | 86.43±0.02 | 78.26±0.03 | 72.49±0.02 |
| 1000 | 95.21±0.03 | 92.34±0.02 | 87.42±0.01 |
| IC50 | 115 | 121 | 249 |
Values are means ± SD (n=5)
Super oxide scavenging activity of methanol extracts of H. intigrifolia
The hydroxy radical is probably the final mediator of most of the free radical induced tissue damages. All of the reactive oxygen species exert most of their pathological effects by giving rise to hydroxy radical formation. Table 3. Showed that the, MHI exhibited concentration dependent scavenging activities against hydroxyl radicals generated in a Fenton reaction. The IC 50 values of MHI, BHT and Q are 275,375 and 450 mg per ml respectively. These results reveal that the MHI exhibiting potent hydroxy radical scavenging activity compared to BHT and Quercetin.
Table 3:
| Concentration (µg) | Percentage of inhibition by | ||
| H. intigrifolia | Butylated hydorxy toluene | Quercetin | |
| 10 | 05.20±0.032 | 03.08±0.032 | 02.31±0.064 |
| 50 | 17.12±0.011 | 13.27±0.084 | 09.41±0.044 |
| 100 | 26.05±0.072 | 25.15±0.015 | 17.02±0.011 |
| 250 | 45.64±0.044 | 41.42±0.056 | 40.25±0.012 |
| 500 | 62.42±0.037 | 58.24±0.068 | 55.64±0.044 |
| 750 | 77.21±0.014 | 67.21±0.013 | 64.21±0.015 |
| 1000 | 96.51±0.012 | 88.30±0.024 | 83.13±0.022 |
| IC50 | 275 | 375 | 450 |
Values are means ± SD (n=5)
Hydroxyl radical scavenging activity of methanol extracts of H. intigrifolia
Table 4:
| Concentration (µg) | Percentage of inhibition by | ||
| H. intigrifolia | Butylated hydorxy toluene | Quercetin | |
| 10 | 18.25±0.032 | 12.08±0.032 | 09.31±0.064 |
| 50 | 23.12±0.011 | 21.27±0.084 | 19.41±0.044 |
| 100 | 32.05±0.072 | 31.15±0.015 | 29.02±0.011 |
| 250 | 49.64±0.044 | 46.42±0.056 | 41.25±0.012 |
| 500 | 72.42±0.037 | 69.24±0.068 | 61.64±0.044 |
| 750 | 77.21±0.014 | 75.21±0.010 | 72.20±0.014 |
| 1000 | 82.30±0.024 | 79.13±0.022 | |
| IC50 | 255 | 275 | 425 |
Values are means ± SD (n=5)
Hydrogen peroxide scavenging activity of methanol extracts of H. intigrifolia
Table 5:
| Concentration (µg) | Percentage of metal chelating rate by | ||
| H. intigrifolia | Butylated hydorxy toluene | Quercetin | |
| 10 | 03.01±0.032 | 02.88±0.032 | 02.36±0.064 |
| 50 | 15.72±0.011 | 12.97±0.084 | 07.47±0.044 |
| 100 | 25.03±0.072 | 24.15±0.015 | 16.72±0.011 |
| 250 | 47.84±0.044 | 46.52±0.056 | 42.25±0.012 |
| 500 | 64.48±0.037 | 58.14±0.068 | 56.84±0.044 |
| 750 | 71.31±0.012 | 60.21±0.014 | 59.21±0.014 |
| 1000 | 77.21±0.014 | 62.89±0.024 | 61.13±0.022 |
| IC50 | 265 | 450 | 47 |
Values are means ± SD (n=5)
Metal chelating activity of methanol extracts of H. intig
The hydrogen peroxide scavenging ability of MHI is given in the table 4, where it is compared with that of BHT and Quercetin as standards. The MHI is exhibiting scavenging hydrogen peroxide in a concentration dependent manner. The correlation between the extract value and those of the control is statistically significant (P<0.05). The MHI, BHT and Quercetin exhibited hydrogen peroxide scavenging activity of 93.90, 82.30 and 79.13% at 1mg/ml with IC50 value of 255, 275 and 425 µg/ml respectively. Although hydrogen peroxide it self is not very reactive, it can sometimes cause cytotoxicity by giving rise to hydroxyl radicals in the cell. Thus, decomposing hydrogen peroxide is very important in biological system.
Metal chelating activity influences the concentration of the catalyzing transition metal ions in lipid peroxidation. It has been reported that chelating agents are effective as secondary antioxidants because they reduce the redox potential, thereby stabilizing the oxidized from of the metal ion (Gordon, 1996). The data shown in the table 5, indicates that the MHI demonstrate a significant iron binding capacity with IC 50 values of 265 µg/ml compared to known antioxidants (BHT 450 µg/ml; Quercetin 475 µg/ml) suggesting the protective action against peroxidation.
Nitric oxide radicals and reactive nitrogen species found to have biological roles in inflammation and in mediating many cytotoxic and pathological events (Darley et al. 1995). Methanol extract of H. intigrifolia exhibited strong nitric oxide scavenging activity (96.60%) in a dose dependent manner which is lower than BHT(98%) and higher than Quercetin (86.62%) at concentration of 1mg/ml (Table 6).
Table 6:
| Concentration (µg) | Percentage of inhibition by | ||
| H. intigrifolia | Butylated hydorxy toluene | Quercetin | |
| 10 | 21.42±0.03 | 8.12±0.03 | 07.52±0.02 |
| 50 | 36.12±0.03 | 32.42±0.05 | 28.41±0.05 |
| 100 | 50.53±0.02 | 49.51±0.01 | 38.47±0.03 |
| 250 | 63.06±0.04 | 62.43±0.03 | 59.62±0.04 |
| 500 | 74.51±0.03 | 72.49±0.02 | 68.49±0.07 |
| 750 | 87.42±0.01 | 84.12±0.04 | 79.12±0.04 |
| 1000 | 98.58±0.02 | 96.62±0.02 | 86.62±0.06 |
| IC50 | 98 | 105 | 225 |
Values are means ± SD (n=5)
Nitric oxide scavenging activity of methanol extracts of H. intigrifolia
Initiation of the lipid peroxidation takes place through hydroxy radical by Fenton’s reaction. Figure 2. shows that the MHI inhibited FeSO4 induced lipid peroxidation of sheep liver homogenate in a dose dependent manner. The inhibition may be caused by scavenging the hydroxyl radical or the superoxide radicals or by chelating the Fe+3 / Fe+2 or by reducing the rate of conversion of ferrous to ferric or by chelating the iron it self. Iron catalyses the generation of hydroxyl radicals from hydrogen peroxide and superoxide radicals. The hydroxyl radical is highly reactive and can damage biological molecules when it reacts with polyunsaturated fatty acid moieties of cell membrane phospholipids and produces hydroperoxides. Lipid hydroperoxides can be decomposed to produce numerous carbonyl products such as malondialdehyde (MDA). The carbonyl products are responsible for DNA damage, generation of cancer and ageing related diseases (Riemersma et al. 2000). Thus the decrease in the MDA level in sheep liver homogenate with the increase in concentration of the extract indicates the role of the extract as strong antioxidant.
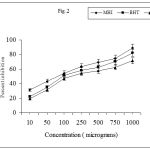 |
Figure 2: Values are means ± SD (n=5).
|
5’-lipooxygenase is innately involved in the inflammation cascade. Cell injury results in the liberation of phospholipids from the surrounding plasma membrane. Phospholipids form arachidonic acid by the action of phospholipase A2. Arachidonic acid can be converted to leukotrienes, which increases oxidant production and can lead to further damage. 5’-lipooxygenase inhibition activity of MHI and its IC50 value is depicted in the table 7. The results indicates that the MHI inhibited 5’-lipooxygenase in dose dependent manner, displaying the most potent activity with an IC50 value of 55.05 mg/ml while NDGA which is represented the positive control, had a value of 60.9 mg/ml.
Table 7:
| Concentration (µg) | H. intigrifolia | Nordihydroguanaretic acid |
| 10 | 21.41±0.21 | 27.34±0.32 |
| 50 | 48.50±0.17 | 49.79±0.32 |
| 100 | 62.45±0.21 | 67.21±0.26 |
| 250 | 71.53±0.28 | 74.44±0.32 |
| 500 | 79.22±0.36 | 81.65±0.31 |
| 750 | 83.36±0.26 | 85.62±0.38 |
| 1000 | 91.34±0.32 | 96.22±0.42 |
| IC50 | 55.05 | 60.90 |
Values are means ± SD (n=5)
5’-Lipoxygease inhibitory activity of methanol extracts of H. intigrifolia
From the overall results of radical scavenging and 5’-lipooxygenase inhibitory activity of H. intigrifolia, it may be concluded that the plant extract has potential antioxidant and anti-inflammatory activities. Further studies on phytochemical analysis are in progress.
Acknowledgements
We thank the GlTAM management for providing the necessary facilities to carry out this work. We also thank Prof. M. Karuna Kumar, Department of Biochemistry, University of Mysore, Karnataka, for providing 5’-lipooxygenase.
References
- Buyukokuroglu ME, Gulcin I, Oktay, M, Kufrevioglu, OI (2001). In vitro antioxidant properties of dantrolene sodium. Pharmacol. Res. 44, 491-95.
- Shahidi F, Wanasundara PD (1992). Phenolic antioxidants. Cri. Rev. Food. Sci. Nutr. 32, 67-103.
- Vaidyaratnam PSV (1994). Indian medicinal plants: a compendium of 500 species, vol.3, 162-163.
- Jayvir A, Parabia M, Bhatt G, Khamar R (2002). A glossary of selected indigenous medicinal plants of India. – SRISTI Innovations, AHMEDABAD- INDIA.
- Benzie IF, Strain J (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal.Biochem. 239,76-81.
- Koleva II, Van Beek TA, Linssen JPH, De Groot A, Evstatieva LN (2002). Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. analy. 13, 8-17.
- Kunchandy E, Rao MNA (1990). Oxygen radical scavenging activity of curcumin. Int. J. Pharmacogen. 58, 237-240.
- Beauchamp C, Fridovich I (1971). Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 44, 276-287.
- Zhang XY (2000). Principles of chemical analysis. Beijing: China Science Press. Pp275-276.
- Garrat DC (1964). The quantitative analysis of drugs. Japan :Chapman and Hall. Vol 3, pp.456-458.
- Dinis TPC, Madeira VMC, Almeidam LM (1994). Action of phenolic derivates as inhibitors of membrane lipid peroxidation and peroxyl radical scavengers. Ach. Biochem. And Biophy. 315,161-169.
- Ohkawa H, Ohishi N, Yagi K (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal.Biochem. 95, 351-358.
- Sircar JC, Shwender CJ, Johnson EA (1983). Soy bean lipoxygenase inhibition by non-steriodal anti-inflammatory drugs. Prostaglandins. 25, 393-396.
- Evans AT (1987). Actions of cannabis constituents on enzymes of arachidonic acid metabolism: anti-inflammatory potentials. Biochem. Pharmacol. 36, 2035-2037.
- Elmastas M, Gulcin I, Isildak O, Kufrevioglu O I, Ibaoglu K, Aboul-Enein HY (2006). Radical scavenging activity and antioxidant capacity of Bay leaf extracts. J. of Iran. Chem. Society, 3 (3), 258-266.
- Gordon MH (1990). In: B.J.P. Hudson (Ed), Food antioxidants. Elsevier-London-New York. pp1.
- Darley –Usmar V, Wiseman H, ( 1995). Nitric oxide and oxygen radicals: a question of balance. FEBS Lett. 369,131-135.
- Reimersma RA, Carruthers KF, Etton RA, Fox K A (2000). Vitamin C and the risk of acute myocardial infarction. Am. J. Clin. Nutr.179(71), 1181-1186.







