S. C. Narwadiya1, U. L. Dhumne2, P. M. Tumane1 and V. G. Meshram3
1P.G. Department of Microbiology, R.T.M Nagpur University Nagpur (India).
²National Institute of Miners’ Health, JNARDDC Campus Opp wadi Police Station Wadi, Nagpur (India).
3Sindhu Mahavidyalaya,Panchpaoli,Nagpur (India).
Corresponding Author E-mail:snarwadiya@gmail.com
Abstract
Increase of liver enzymes e.g. LDH and SGPT are common in a tuberculosis regimen. Patients taking anti - TB drugs develop severe hepatitis that may progress to liver failure and death if drugs not stooped promptly (Timberel JA, 1985). Dots chemotherapy in India, treatment of category is divided into 3 types- Category I, II and III. Hepato-toxicity in TB therapy has been well documented but the present study had objectives to assess the liver enzymes according to category of treatment. A total 90 patients were taken in different category of treatment from dot center and analyze SGOT, SGPT and alkaline phosphatase level with respect to detail data was recorded for each and every patient. Study found that significant abnormal rise of liver enzymes in category of treatment II and the values came to near normal in category of treatment III. Due to different drug doses in different category. Study results comparatively related with some other study.
Keywords
Liver Enzymes; TB regimen; Assessment
Download this article as:| Copy the following to cite this article: Narwadiya S. C, Dhumne U. L, Tumane P. M, MeshramV. G.Assessment of Liver Enzymes Level in the Serum of Tuberculosis Patientsduring Directly Observed Short Term Chemotherapy (DOTS).Biomed. Pharmacol. J.2008;1(2) |
| Copy the following to cite this URL: Narwadiya S. C, Dhumne U. L, Tumane P. M, MeshramV. G.Assessment of Liver Enzymes Level in the Serum of Tuberculosis Patientsduring Directly Observed Short Term Chemotherapy (DOTS).Biomed. Pharmacol. J.2008;1(2). Available from: http://biomedpharmajournal.org/?p=507 |
Introduction
Directly observed treatment short course (DOTs), based on WHO framework was introduced in India for effective control of tuberculosis. Isoniazid (INH), rifampicin (RMP), pyrazinamide (PZA), ethambutol (E), streptomycin (S) has been successful therapeutic agents for the treatment of TB because of high therapeutic efficacy and good acceptance. All anti-tuberculosis drugs with the possible exception of streptomycin can cause hepatitis but the risk is higher with some other drugs than with others (Girling, 1978). Identification of patients at increased risk for anti-TB drugs-induced hepatotoxicity is important because hepatotoxicity causes significant morbidity and mortality and may
require modification of drug regimen. However, factors predicting anti-TB drugs-induced hepatotoxicity is still controversial. Hepatic damage is assessed at the earliest with the help of clinical laboratory by various liver function tests. Hepato-toxicity due to Rifampicin and Pyrazinamide has been reported by McDermott et al.,(1954), Philips and Horten (1956), Macleod et al., (1959), USPHS (1959) and Lees et al., (1970). Retreatment regimen consisting of Rifampicin and Ethambutol in cases where primary chemotherapy has failed has been reported to carry a very low risk of hepatitis (Girling, 1978). Initial supplement of another drug, preferably bactericidal, can give us 100% success rate with tolerable minimal side effects in re-treatment regimen. The present study was conducted to assess liver enzymes according to category of treatment during tuberculosis regimen and which category more chances of hepatic damage by measuring their serum enzymes level.
Material and Methods
The study was conducted in the DOTs center in Nagpur. The study comprises a total 90 patient with pulmonary tuberculosis infection. There were 63 (70%) male and 27 (30%) female, with ages ranging 23 to 63 years (mean 46.9 ± 9.46 years). A brief patients data was recorded with respect to age, sex, food , smoking , alcohol habit, personal and family history, present and past medical status, symptoms etc. Along with this data, height in centimeter and body weight in kilo-gram was doneto calculate body mass index. (Table1). They were negative for HBsAg, anti-HCVAb, and HIV. Liver function was monitored by measuring the serum level of AST, ALT and alkaline phosphatase with the help of an auto-analyzer in the pathology lab. Liver enzymes were estimated according to completion of category of treatment by the following methods. ´ Estimation of SGOT and SGPT (Retimans and Frankel, 1957).
 |
Table 1: Baseline demographic characteristics of TB patients.
|
Estimation of serum alkaline phosphatase (Wootton, 1964). Data analysis was performed by using statistical software Epi Info version 6.00.
Results and Discussion
Data are presented as mean±SD except sex, smoking habit, alcohol habit, clinical, history and family history. Table 1 shows baseline demographic characteristics of study TB patient. Out of total study subjects there were 63 male and 27 female, with age of mean 46.9 ± 9.46 years. 65.5 % and 56.6 % TB patients were having smoking and alcoholic habit respectively while 34.4 % and 43.3 % subjects no any habit. Body mass index of study patients were low (less than 20 kg/cm².). History of tuberculosis patients like gastrointestinal manifestation, weight loss, loss of appetite such as clinical history noted in 81 study subjects. Out of 90 subjects 8 subjects having past family history. According to past history, it was noted that 8 subjects there were suffering tuberculosis infection from earlier tuberculosis patient in his family. Results of serum enzymes after each category of treatment shown in table 2. Results show in mean and standard deviation. Mean serum AST, and HIV. When study subjects in intensive phase among category of treatment I that period serum sample were collected and estimated. Same collection and estimation were done among next category. It was concluded that more chances of hepatic damage during tuberculosis regimen in category of treatment II. Study results found there were no risk in category of treatment III cause of these rise enzymes level come to near normal range.
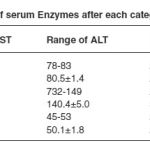 |
Table 2: Level of serum Enzymes after each category of treatment.
|
Acknowledgments
The authors are grateful to the Dots staff, Patients and to the people who are direct and indirect help, made this study. ALT and alkaline Phosphatase level found in category I was high just above the normal level meanwhile results found in category II is much more high as compare to category I and normal range. Increased enzymes level in category II was there reach to normal in category III. Study results found that chances of Hepatotoxicity seen in category II due to high increased serum enzymes level.
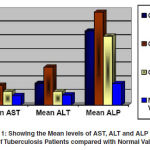 |
Graph 1: Showing the Mean levels of AST, ALT and ALP of serum of Tuberculosis Patients compared with Normal Values.
|
Conclusion
90 known cases of pulmonary tuberculosis were studied for assess the liver enzymes among them. They were getting treatment according to dots chemotherapy. On initiation of the treatment they were confirmed negative for HBsAg, anti-HCVAB
References
- Lees, A. W., Asgherb, Sinha, B.N.:Jaundice after rifampicin. Br. J. Dis. Chest., 64: 90 (1970).
- Ban, B.: Hepatic damage in chronic pulmonary tuberculosis. Am. Rev. Tuberc., 72: 71 (1955).
- Allison, S.T., Pyrazinamide and Isoniazid in low dosage in treatment of pulmonary tuberculosis. Am. Rev. Tuber. 4: 460 (1956).
- Matthews, J.H., Pyrazinamide and isoniazid used in the treatment of pulmonary tuberculosis. Am. Rev. Resp. Dis. 81: 348 Narwadiya et al., Biomed. & Pharmacol. J., Vol. 1(2), 449-452 (2008) 451 (1960).
- Guidelines for the management of adverse drug effects of antimycobacterial agents, Lawrence Flick Memorial Tuberculosis Clinic, Philadelphia TB Control program (1998).
- Timbrel, J.A., Park B.K., Harland S.J., A stuffy of the effects of RMP on INH metabolism in human volunteers. Hum Toxicol, 4: 279-285 (1985).
- Koponoff D.E., Sinder D.E. Jr, Caras G.J., INH-related hepatitis: a US-public health service co-operative rveillance study. Am Respir Dis, 117: 991-1001 (1978).







