Aara Rifat, S. A. Hajam and K. I. Andrabi
Department of Biochemistry, University of Kashmir, Kashmir -190 006 (India).
Abstract
Heat stability refers to the fraction of proteins that remain soluble when heated to 90°C thus greatly facilitating their enrichment subsequent purification. Under these conditions approximately 95% of the total cell protein (heat stable) fraction from sheep liver homogenate was thus prepared and analyzed to meet the objectives of the study.The thermolabile proteins were removed by centrifugation and supernatant containing soluble proteins were stored until processed further. At least proteins were purified to homogeneity using conventional biochemical techniques of salt fractionation with ammonium sulfate, followed by DEAE-Cellulose chromatography and the purity analyzed by SDS-page.
Keywords
HSP Salt fractionation; SDSpage
Download this article as:| Copy the following to cite this article: Rifat A, Hajam S. A, Andrabi K. I.Analysis and Partial Purification of Heat Stable Proteins in Sheep Liver Homogenate by Salt Fractionation Using Ammonium Sulfate.Biomed. Pharmacol. J.;1(2) |
| Copy the following to cite this URL: Rifat A, Hajam S. A, Andrabi K. I.Analysis and Partial Purification of Heat Stable Proteins in Sheep Liver Homogenate by Salt Fractionation Using Ammonium Sulfate.Biomed. Pharmacol. J.2008;1(2). Biomed. Pharmacol. J.2008;1(2). Available from: http://biomedpharmajournal.org/?p=512 |
Introduction
Several polpeptides are known as heat or stress inducible proteins in both eukaryotes. They are commonly refered to as heat shock proteins(hsp) and are highly conserved throughout evolution (Schlesinger et al1982, Atkinson and Walden 1985, Pardue et al 1989).Significant amounts of these hsps are present in cells even in the absence of stress. These hsps perform basic and indispensable cellular functions at normal growth tempreture in addition to protecting cells from stress related deleterious effects.The hsp70 family and hsp60 have been shown to have a molecular chaperoning activity, involving them in the folding and assembly of nascent proteins and in their translocation across the membranes of the endoplasmic reticulum and mitochondria(Chirico et al 1988, Deshaies et al 1988, Cheng et al 1989, Beckman et al 1990, Pelham 1984).
Methodology
Preparation Of Crude Liver Extract
Frozen sheep liver was cut into small pieces then homogenized in lysis buffer containing (10mM Tris acetate pH=7.5, 10mM NaCl, 1mMEDTA, 1mMPMSF) using a hand held homogenizer. The homogenate was centrifuged at 700g for 30 minutes Pellet was discarded and the supernatant recentrifuged at 700g for 30 minutes. The supernatant were saved as total cytosolic protein extract.
Preparation Of Boiled Extract
Crude extract prepared as was incubated at 950C water bath for 7-10 minutes with constant stirring and cooled on ice. The precipitated protein was discarded following centrifugation and the remaining supernatant was saved as heat stable fraction.
Ammonium Sulfate Fractionation
The heat stable fraction obtained was subjected to differential precipitation using ammonium sulfate at 40%-90% saturation by slow addition of calculated quantities of solid ammonium sulfate at each step to the supernatant (heat stable fraction from liver extract) with constant stirring using a magnetic stirrer at 40C. After obtaining the desired saturation of ammonium sulfate at 40%, the suspension was kept at 40C for 30 minutes to enable protein precipitation. The precipitated protein was pelleted down by centrifugation at 13000 rpm for 10 minutes. Pellet was suspended in a minimal amount of buffer (10mM Tris acetate pH=7.5, 10mM NaCl, 1mM EDTA). Supernatant was used to precipitate the proteins at subsequent higher saturation of ammonium sulfate i,e 50% -90%. The pellet obtained at each step was suspended in a minimal amount of buffer A and dialyzed against the same buffer [400ml] at 40C for 24 hours. Continue this procedure to produce 40% pellet, a 50% pellet, a 60% pellet, a 70% pellet, a 80% pellet, a 90% pellet and a 100% supernatant.
Deae-Cellulose Choromatography
Dialyzed ammonium sulfate fractions were applied separately on to a DEAE-Cellulose column {4cm ´2mm} equilibrated with buffer A (20mM Tris acetate pH=7.6, 20mM NaCl, 0.1mMEDTA). After washing the column with buffer A until the absorbance of the eluate decreased to less than 0.025 at 280nm. Mixture of proteins bound as a yellow zone at top of column was eluted with a linear 10mM-500mM NaCl gradient in buffer A at a flow rate of 25ml/hour. Gradient volume used was 5 times the bed volume of mini column [4ml]. Fractions of 0.5ml of the eluate collected. Same procedure applied for every dialyzed salt fractionated sample.
Protein estimation
Protein concentrations at each step of the purification was determined by bradford method using bovine serum albumin as standard.
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis
SDSpage was carried out in 12% separating gel with a 5% stacking gel according to lammilli. The proteins were visualized by staining with 0.1% coomasie brilliant blue R250.
Result
The heat stable fraction was fractionated by ammonium sulfate from 40%-90% saturation respectively. The original volume of the sample taken was 30ml and calculated quantities of salt was added according to the formula
533(S2-S1) ×1000
100-0.3×S1
Where S1= initial salt concentration and S2=final salt concentration added. At 30% saturation with ammonium sulfate no pellet is obtained. After 40% saturation, 50% 60% 70% 80% 90% pellets obtained are then dissolved in minimal amount of buffer[Tris acetate pH=7.5] and then dialyzed against the same buffer. Protein concentration at each step of the purification was determined by Bradford method using bovine serum albumin as a standard.
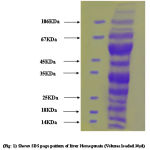 |
Figure 1:
|
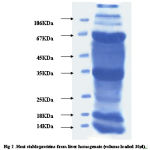 |
Figure 2:
|
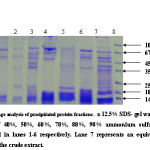 |
Figure 3:
|
 |
Table 1: Cellular fractionation of tissue.
|
Protein concentration at each step of the purification was determined by the Bradford method using bovine serum albumin as a standard. Samples from different salt precipitated pellets were taken and preserved for protein estimations and SDSpage analysis.Coomasie stain shows the constituent bands of mol.wt. Ranging (100KDa-45KDa) and (40KDa-25KDa).
An identical pattern was seen at different saturation of salt in some lanes. Further purification of major heat stable proteins was achieved by subjecting each of the dialyzed fractions to DEAE-anion exchange chromatography. A mini column of (4ml) bed volume was equilibrated with buffer containing (20mM Tris- acetate pH7.6, 20mM NaCl, 0.1mM EDTA). The unbound proteins were collected as flow through 2-3 washes with 2.5 column volumes ensured complete removal of unbound protein. The absorbed proteins were eluted with continuos NaCl gradient (10mM-500mM) for about 3 bed volume worth of eluates in buffer A at flow rate of 25ml/ hr. 50 fractions of 0.5ml each were collected. An aliquot of every third fraction was analyzed by SDS-page for corroboration with absorbance pattern.
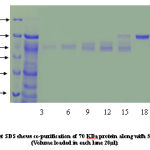 |
Figure 4:
|
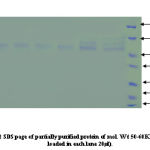 |
Figure 5:
|
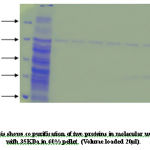 |
Figure 6:
|
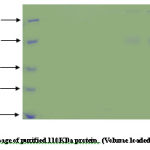 |
Figure 7:
|
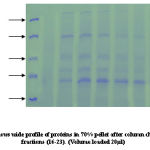 |
Figure 8:
|
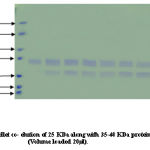 |
Figure 9:
|
 |
Figure 10:
|
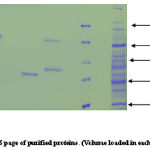 |
Figure 11:
|
Co-purification of proteins 40% active dialyzed fraction of 1ml volume when applied to anion exchange chromatography. 70KDa protein co purifies with protein of molecular weight nearly 50-60KDa in fractions (8-15) these were not further seperated because 57KDa protein was present in very low concentration. In 50% pellet 70 KDa fractions (15-22) and 57KDa protein fractions (30-35) obtained seperately when applied to column. Wide profile of proteins found in 70% pellet. In 90% sample again co-purification of proteins take place. Protein of 35-40KDa mass co-purifies with 25KDa protein fractions [28-32]. We attempted to saperate 25KDa from majority of 35-40KDa protein. For this longer salt gradient (400-500mM, 8 times bed volume) was used and fractions of 1ml volume collected. 25KDa protein eluted in the 11th fraction after DEAE-Cellulose column chromatography.
References
- Atkinson, B.G and Walden, D.B., eds (1985).Changes in eukaryotic gene expression in response to en
- Cheng, M.Y., Hartl, F-U., Martin, J. Pollock., R.A., Kalousek, F. Neupert, W., Hallberg, E.M., Hallberg, R.L., and Horwich, A.L. The mitochondrial chaperonin Hsp60 is required for its own assembly. Nature (1989) 337, 620-625.
- Pardue, M.L., Feramisco, J.R. and Lindquist, S. eds. Stress induced proteins. (1989) Alan R.Lss. Inc.
- Deshaies, R., Koch, B., Werner- Wash burne, M., Craig, E.A., and Scheckman, R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptide. Nature (1988) 332, 800-805.
- Beckmann, R. P., Lovett, M., and Welch, W.J. Examining the function and regulation of Hsp70 in cells subjected to metabolic stress. J. Cell Biol.( 1992) 117, 1137 – 1150.







