A. Thirupathaiah¹*, G. Venkateshwar Rao¹ and Yoshihisha Takaishi²
1Department of Chemistry, Kakatiya University, Warangal,India.
²Faculty of Pharmaceutical Sciences, University of Tokushima, Somachi-1-78, Tokushima,Japan.
Abstract
From the petroleum ether extract of roots of Crossandra nilotica, a furofuran type of symmetrical lignan named crossandrin (1) was isolated. The structure was established by spectroscopic techniques like 1H, 13C NMR, Mass and 2D NMR screened for antimicrobial activity.
Keywords
Crossandrin; Crossandra nilotica; Acanthaceae; symmetrical lignan; antimicrobial activity
Download this article as:| Copy the following to cite this article: Thirupathaiah A, Rao G. V, Takaishi Y. A lignan from the roots of Crossandra nilotica.Biomed Pharmacol J 2008;1(2). |
| Copy the following to cite this URL: Thirupathaiah A, Rao G. V, Takaishi Y. A lignan from the roots of Crossandra nilotica.Biomed Pharmacol J 2008;1(2). Available from: http://biomedpharmajournal.org/?p=435 |
Introduction
The Crossandra nilotica belongs to the family Acanthaceae. It is a shrub and occur in Andhrapradesh, Kerala, Tamilnadu. This plant has pre-emptive treatment with wide range insecticide and with a systemic fungicide to prevent the attack from part of the aphids and the development of fungus disease, often favored by a mild and damp climate. There is no phytochemical report on this plant, this presentation describes the extraction, isolation, structure elucidation and antimicrobial activity of crossandrin from the roots of Crossandra nilotica plant for the first time, which was earlier reported as ecbolin B from the Ecbolium linneanum1.
Reults and Discussion
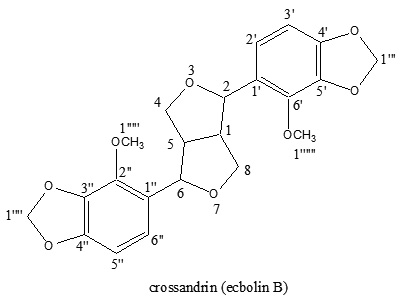
The petroleum ether extract of the roots of the Crossandra nilotica was directly yielded a symmetrical furofuran type lignan. The structure was established by physical and spectroscopic analysis. The HRMS spectrum of crossandrin (1) indicated that the molecular weight of 414.41 corresponding to the molecular formula C22H22O8.The molecular ion peak M+ occurred at m/z 414.41 and the base peak at m/z 179. The other significant peaks were appearing at m/z 233,208 and 152. The UV spectrum of compound (1) showed maxima 238 and 275 nm which corresponds to the furofuran type of lignan. In IR spectrum of the compound (1) taken KBr pellets exhibited bands at 1630,1615,1468,1108 cm-1 which also in complete agreement with furofuran type of lignan2, the peak at 1108 cm‑1 corresponds to O-C-O stretching; aromatic C-H stretching frequency appears at 1433 cm-1. 1H NMR (Table 1) signal pattern in the aromatic region suggested that the presence of two doublets at d 6.53 and d 6.51 respectively3,4. It also showed 6H singlets at d 3.96 indicating the presence of two –O-CH3 groups. The two peaks at d 5.97 and d 5.94 corresponding to O-CH2-O groups. The COSY and HMBC spectra (Table 1) were also in support of the structure of crossandrin. In the long range COSY correlations d 6.82 (1H,d,J=8.10 Hz)is interacting with 4.75(1H,d,J=6.6Hz); 5.25(1H,d, J=6.4 Hz) is interacting with 3.35(1H,m);4.75(1H,d, J=6.6 Hz) is interacting with 3.12(1H,m); 3.12(1H,m) is interacting with 5.25(1H,d, J=6.4 Hz).
In HMBC correlations (Table 1) d 54.2 shows cross peak with d 3.03, d 4.75, which indicates that the connectivity of carbon at d 54.2; d 81.9 shows crosspeak with d 5.25, and d 6.5; d 148.7 shows crosspeak with d 6.82, d 5.97 and d 6.50. 13C NMR (Table 1) data also confirmed the structure of the compound (1), the aryl carbon atoms of one ring were assigned with d 136.33(C-1¢ & C-1¢¢), d 106.4(C-2¢ & C-2¢¢), d 140.79(C-3¢ & C-3¢¢), d 148.73(C-4¢ & C-4¢¢), d 118.7(C-5¢ & C-5¢¢), d 127.3(C-6¢ & C-6¢¢). The methylene dioxy carbon atoms were assigned with d 102.24. The two methoxy carbon atoms were assigned with d 59.48. The crossandrin (1) is a symmetrical due to the no change in the values of aryl carbon atoms(Table 1). On the basis of Mass,UV,IR, 1H NMR, COSY,HMBC and 13C NMR spectral data the structure was established for crossandrin and the name is 5-[hexahydro-1-(4-methoxybenzo [d] [1,3] dioxol-5-yl)furo[3,4-a]furan-4-yl)]-4-methoxybenzo [d] dioxle. Crossandrin is the first phytochemical report of Crossandra nilotica.
Table 1. The 1H, 13C NMR, NOESY and HMBC spectral data for crossandrin (1).
| Position | 1H (J in Hz) | 13C | NOESY with H at | HMBC with at |
| 1,5 | 3.12(1H,m) | 54.20 | 2,4,6,8 | 1,2,4,8 |
| 2,6 | 4.75(1H,d,J=6.6) | 73.30 | 6¢,6¢¢,8 | 6¢,8 |
| 4,8 | 3.03(2H,m) | 72.60 | 2,6,5 | 5,6 |
| 6 | 5.25(1H,d, J=6.4) | 81.90 | 6¢¢,5 | 4,5,1,6¢¢ |
| 1¢, 1¢¢ | – | 136.33 | – | – |
| 2¢, 2¢¢ | – | 106.40 | – | – |
| 3¢, 3¢¢ | – | 140.79 | – | – |
| 4¢, 4¢¢ | – | 148.73 | 5¢,6¢,5¢¢,6¢¢ | 5¢,6¢,5¢¢,6¢¢ |
| 5¢, 5¢¢ | 6.82(1H,d, J=8.1) | 118.57 | 6¢,6¢¢ | 5¢¢,6¢¢,5¢,6¢ |
| 6¢, 6¢¢ | 6.50(1H,d, J=8.1) | 127.30 | 5¢,5¢¢ | 1¢,5¢¢ |
| 1¢¢¢, 1¢¢¢¢ | 5.97(2H,s) | 102.24 | – | – |
| 1¢¢¢¢¢, 1¢¢¢¢¢¢ | 3.97(6H,s) | 59.2 | – | – |
Crossandrin(1) was evaluated for antimicrobial a ctivity5,6(antibacterial and antifungal ) against two gram-positive bacteria( Staphylococcus aureus and Bacillus subtilis) and two gram-negative bacteria fungi (Asperigillus paraciticus and Candida albicans). Crossandrin(1) exhibited more activity against both the organisms(i.e., gram-positive and gram-negative organisms and more potent against Candida albicans than Asperigillus paraciticus (Table 2 & 3). How ever it does not show antimicrobial activity at higher concentrations.
Table 2: Antibacterial activity of crossandrin (1).
| Compound | Dose in mg | Organisms and zone of inhibition in mm | ||||
| Gram positive | Gram negative | |||||
| Staphylococcus aureus | Bacillus subtilis | Escherichia coli | Proteus vulgaris | |||
| crossandrin | 100 | — | — | — | — | |
| 50 | — | — | — | — | ||
| 33.3 | 14.0 | 12.0 | 12.0 | 11.0 | ||
| 16.7 | 16.5 | 14.5 | 15.0 | 13.0 | ||
| Streptomycin | 20 | 20.0 | 18.0 | 18.0 | 20.0 | |
| Ampicillin | 20 | 200 | 22.0 | 18.0 | 17.0 | |
Table 3: Antifungal activity of crossandrin (1)
| Compound | Dose in mg | Organisms and zone of inhibition in mm | |
| Aspergillus parasiticus |
Candida albicans |
||
| crossandrin | 100 | — | — |
| 50 | — | — | |
| 33.3 | 6.0 | 8.0 | |
| 16.7 | 10.5 | 17.0 | |
| Clotrimazole | 10 | 18.0 | 27.0 |
Experimental
Crossandra nilotica was collected and identified from premises of central library, Kakatiya University, Warangal. A.P. India in November 2003 by Prof. V. S. Raju, Department of Botany, Kakatiya University, Warangal, A.P. India. Voucher specimen (references Rao-56) have been deposited in the Chemistry Department herbarium. The air dried root material(200 g) was ground into fine powder, this powder material was extracted with petroleum ether (bp 60-80 0C) (2.5 Lx3) in soxhlet extractor for about 72 hours. The petroleum ether extract was concentrated under reduced pressure. On long standing the solid separated in extract, that was filtered and dried under vaccum pump to give pale yellow needles. It was gave a single spot on TLC plate (3:1 petroleum ethr – chloroform). It was recrystallised from chloroform (100 mg.[yield 0.05%] m.p. 101-103 0C).
Acknowledgemwnts
The authors are grateful to Prof. M.A. Singaracharya and K. Vasu, Department of Microbialogy, Kakatiya University, Warangal. A.P. India for their antimicrobial assay and Prof. V.S. Raju, Botany Department, Kakatiya University, Warangal.A.P.India for the authenticfication and collection of the plant material.
References
- Venkataraman, R. and Gopalakrishna, S. Asain,J.Chem 14(1),2772-2282(2002)
- Janesh, M., Jakupovic,J., King,R.M. and Robinson,H Phytochemistry 28(12), 3497-3501(1989)
- Ayres,D.C. and Loke,J.K Lignans-Chemical, Biological & clinical Properties, Cambridge University Press,209-213(1990)
- Abe, F., Yamuchi, T. Phytochemistry 28(6),1737-1741(1989)
- Macraew, W.D. and Towers, G.H.N. Phytochemistry 23(6),1207-1220(1984)
- EI-Egami,A.A., AI-Magboul,A.Z. and Omar Tohami,M.S. Fitoterapia 59(4),369(1998)
- Tanira, M.O.M., Bashir,A.K., Dib.R., Goodwin,C.S. and Wasfi, A. J.ethnopharmacol. 4(3),201(1994)