J. K. Mapko and G. A. Amuga
Department of Biological Sciences, Nasarawa State University, Keffi Nigeria
Abstract
The performance of Simulium hargreasvesi larvae with regards to pupation success and pupal emergence at two temperature regimes were investigated. S. hargreavesi larvae showed better success at the higher temperature of 23 0C with a mean percentage pupation index of 30.0 and a mean percentage survival index of 619.5. The mean percentage pupation index at 19 oC was 21.7 with a mean survival index 0f 511.2 suggesting that high temperatures favour survival and pupation. It is therefore apparent here that high temperature and flow rates favour the development of the blackfly larvae in the laboratory. These observations can be useful in developing fly control strategies in Africa.
Keywords
Variations; transition; pupae; Simulium hargreavesi; temperature
Download this article as:| Copy the following to cite this article: Mapko J. K , Amuga G. A. The Effect of Temperature on the Survival of Simulium Hargreavesi Larvae Reared in the Laboratory. Biomed Pharmacol J 2008;1(1). |
| Copy the following to cite this URL: Mapko J. K , Amuga G. A. The Effect of Temperature on the Survival of Simulium Hargreavesi Larvae Reared in the Laboratory. Biomed Pharmacol J 2008;1(1). Available from: http://biomedpharmajournal.org/?p=195 |
Introduction
Simullids are cosmopolitan, occurring in wide range of geographical areas where there is running water of sufficient permanence to provide the habitat requirements of the juvenile stages. Wilson et al (2002) reported that blackflies can occur at very localized sites even in hot deserts where natural water courses or irrigation channels exist, and that some species survive in the melt-water below glaciers, but at very high latitudes the number of species is small and blood-sucking species few or non.
The immature stages (larvae and pupae) are generally found in fast flowing fresh water courses ranging from the merest temporary trickles to the greatest rivers, though each species tends to prefer one particular type of habitat. This type of distribution has been explained on the basis of high oxygen concentration (Wilson et al 2000) as it especially affects the pupae and current speed (Awadzi et al 2004). Blackfly populations have been reported by Krueger(2001) and other workers from various parts of Nigeria, including the Jos Plateau, about 30000 meters above sea level and parts of Nasarawa State. Their roles as vectors in the transmission and maintenance of the nematode infection, onchocerciasis with its medical and economic implications in tropical Africa have been enumerated (Usip et al, 2006).
It has been suggested (Mafuyai et al, 1994) that in natural populations of Simulium spp, the responsiveness of the rates and degree of larval development to transient abnormal temperatures or dramatic drop in food availability play a major role in regulating variations in population abundance rather than mean seasonal values of these factors. Different temperature ranges have been reported for the S. damnosum complex by pioneer workers in West Africa (Qullerere et al, 1976; Grenwald, 1977). They observed that the immature stages of S. damnosum and S. sirbanum develop at water temperatures ranging from 240c to 330c with mean value of 270c.
Materials and Methods
Samples of the blackfly S. hargreavesi larvae were collected from the upper course of River Assob at the fringes of the Jos Plateau about 64km along Jos-Akwanga Road. The river is characterized by fast current velocity, small branching channels, ripples and pools and a rocky bed. The larvae were found mainly on trailing vegetation, submerged rocks and dead leaves in fast-flowing parts of the rivers with current velocity ranging from 1 to 2.6ms-1.
The sampling area was divided into upper (U), Middle (M) and lower (L) courses characterized by mean flow rates of 1.3m/s, 1.1 m/s and 2.6m/s respectively. The average depths were 44cm, 33cm and 28cm in that order.
Samples were collected into polythene bags and transported to the laboratory while still attached to the substrates. Identification and separation of the larvae into different species were carried out in the laboratory immediately on arrival from the field. Three different species were identified during this study namely: S. cervicornutum, S. vorax and S. hargreavvesi.
Breeding was carried out in an aquatron, which consisted of a continuous water circulation system comprising an elevated water tank from which water flowed through a fine filter into three taps. Water was then tapped from one of the three taps into an aquatron designed to accommodate three rearing tubes at three contrasting gradients of 00, 200 and 420. The water inlet was by a main delivery line branching off at appropriate points corresponding to the gradients. The current velocities for these gradients were 13.6cms-1, 25.8cm/s ad 46.5 cm/s respectively.
The rearing tubes had an equal bore diameter of 1.23cm while the aquatron was 31cm by 42cm in size.
A Gessner current meter was used to measure the water current velocity in cms-1 and calculated by the method of Wilson et al (2000) as:

Three groups comprising a specific number of larvae per group were reared at a controlled temperature of 190C. S. hargreavesi larvae were attached to three polythene strips and one was introduced into each of the three tubes in the aquatron. The water delivery tap was then opened slowly to allow the larvae get attached to the polythene firmly to avoid washing them off.
The larvae were fed with a suspension of algae collected from an artificial pond, centrifuged and introduced at the inflow part of the circulating water using a hypodermic syringe.
Temperature and other physico-chemical parameters such as dissolved oxygen, PH., conductivity and turbidity were monitored twice daily. Water was taken at5 the inflow and out flow points to monitor these physic-chemical parameters of interest. Records of the parameters were taken using a Horiba Model U-7 water checker.
The larvae were introduced into the rearing system at a temperature of 19 0C after which it was raised and maintained at the desired level using a water heater. Data were obtained on: (a) duration of pupation (b) number of larvae surviving after each day (c) emergence of pupae to imago at the two temperature regime.
Results
The data on water quality characteristics monitored in the study are shown on table 1. The data indicate variation in dissolved oxygen (DO) levels at the two temperature regimes (190C and 230C).
The table shows decreasing percentage DO differential with increasing temperature from 190C. This is as a result of the little amount of oxygen, which dissolves as temperature goes higher. The PH value does not seem to vary much as can be seen from the data. At 230 there was a proportionate increase in both DO and temperature.
Observations on survival and pupation at 190C and 230C are presented on table 2. The survival index is higher at 230C temperature regime with a cumulative mean percentage of 631.5 S.E ± 12.7 than at 190C with a mean value of 511.2. The mean percentage pupation showed a value of 21.7 as the total survival index at 190C. At the 230C the mean percentage transition showed 30.0 as total pupation index (Table 3).
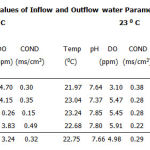 |
Table 1: Average Values of Inflow and Outflow water Parameters at 190C and 230C
|
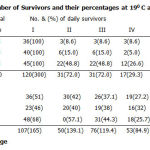 |
Table 2: Number of Survivors and their percentages at 190 C and 230C |
 |
Table 3: Percentage daily pupation at 190C and 230C |
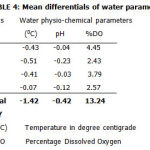 |
Table 4: Mean differentials of water parameters |
Discussion
In both the control and experimental conditions, observations on water quality indicated less DO at high temperatures and more at lower temperatures. This is because more oxygen dissolves at lower than at higher temperatures. It was also noted that there were little variations in pH and conductivity values. pH value remained at 7±1 throughout the period of the experiment. This confirms little variation in the water condition from sometimes acidic to neutral and alkaline water. The little variation observed could be attributed to the waste products from the larvae and other organisms that are in the rearing water, which could not be filtered out completely. On the changes in conductivity levels, there is a probability of up-take and out-put of ionized salts by the larvae. Most of the parameters monitored in the laboratory during this experiment showed more or less similar conditions with that of the natural habitat of this blackfly, Simulium hargreavesi. Mbah et al (2003) reported that different blackfly species had differences in their breeding sites with regards to the chemical composition of water especially with regard to pH and conductivity.
Computation to find out larval survival for the control and the actual experimentals respectively, showed mean total survival indices of 511.2 in the former as against 619.5 S.E ± 12.7 in the latter. This is an indication that survival was enhanced at the higher temperature of 230C more than at 190C. This result seems to tally with the observations of Mbah et al (2003) and Ralph (2004) who noted that the most important physical factor regulating blackfly larval development was temperature.
They observed that this variable played a major role in determining hatching, pupation and emergence, and was responsible for the timing and duration of the life cycles of each species. Shelly et al (1995) observed that temperature affects the number of simulid species in a stream and the life cycles of their parasites. Colbo and Porter (1981) observed that larvae at the lowest tolerable temperature range develop slowly but also requires a considerably reduced concentration of food in the water to complete development and reach maximum size compared with the same species at a higher temperature. In addition, a marginal habitat in terms of food supply at cold temperatures may lead to starvation at even slightly higher temperature (Hadis et al 2005). From the foregoing, it can be said that low temperatures have negative effects on the survival of the larvae of simulids. Table 4 shows negative temperature and PH values due to the fact that the temperature of inflow water was lower than outflow water because of the temperature regulation using a heater in the aquatron.
In experiments similar to this study, Wilson et al (2000) observed that temperature profoundly affected the rate of larval growth and survival of S. venustum Say. They reported a steady decrease in survival rate at lower temperatures, until 120C below which there was no single survivor. Maximum survival was recorded at 220C while at 240C, survival rate dropped. This reveals that in a tropical climate like Nigeria, survival could be much higher above the 230C used in this study. Until the blackfly reaches an upper thermo-tolerance limit. On the other hand, 190C, which was the control temperature, could be considered low and hence it gave lower survival similar to the 120C and below in a temperate climate.
Survival was found to decrease with increase in time from the commencement of each experiment in both control and the experimentals. Since at the end of study period larvae survived better at 230C than at 190C, it could be said that rapid growth, which is obtained at high temperatures, enhances survival. The duration of this experiment took an average of 6 days at 230C. Transition was observed to occur during the first 4-5 days. The results show that transition from larvae to pupae was better at 230C, than at 190C. This could be attributed to the important effect of temperature on the development of the blackfly larvae as earlier mentioned. In the two experiments at two temperature regimes, it was observed that the highest temperature did better in enhancing both survival and pupation. This is probably as a result of the fact that blackfly larvae occur in fast flowing water with reasonably high current velocity and favorable conditions for adequate oxygen up-take by the larvae. The use of a water pump to maintain a continuous water circulation consequently favoured survival and perhaps increased the efficiency of food intake by the larvae.
General observation on emergence from pupae to imago showed that most of the larvae could not emerge as adults after pupation. It is probable that this could have been as a result of the fact that the rearing tubes offered no access to the atmosphere to allow exposure to the mechanism that trigger emergence. Total emergence was slow but this could not be linked to any of the factors on which basis survival and transition have been explained. These findings are useful in blackfly control and reduction of morbidity and mortality due to onchocerciasis.
References
- Awadzi K., Attah S. K., Addy E. T., Opoku N. O., Quartey B. T., Lazdins-Helds
- J.K., Ahmed K., Baotin B. A., Boakey, and G. Edwards., (2004). Thirty-month followed-up of sub-optimal responders to multiple treatments with ivermectin, in two onchocerciasis – endemic foci in Ghana. Annals of Tropical Medical Parasitology 98(4): 359-370.
- Hadis M., Wilson M. D., Cobblah M., and Boakey D.A., (2005). Cytotaxonomic description of Simulium kaffaense, a new member of the S. damnosum complex (Diptera: Simulidae) from South-Western Ethiopia. Annals of Tropical Medical Parasitology. 99(3): 267-291.
- Krueger, A. (2001). Guide to blackflies of the Simulium damnosum complex in Tropical Africa. Medical and Veterinary Entomology 20(1): 60-75.
- Mafuyai, H. B., Philips A., Molyneux, D. H., and Milligan, P. (1994). Identification of Larvae of the Simulium damnosum complex from Nigeria by analysis of Cutiular hydrocarbons. Tropical Medical Parasitology 45(2): 130-132.
- Mbah, C.E., Nock, I.H., and Vajime, O.G., (2003). Laboratory rearing and distribution of the breeding sites of Simulium species in Kaduna state. Journal of Aquatic Sciences 18(2): 131-140.
- Ralph, S. (2004). Field studies on exposure, effects and risk mitigation of aquatic Non-point sources insecticides pollution. Journal of Environmental Quality 33:419-448.
- Shelly, A.J., Luna-Dias A.P.A., Mai-Heszog, M., Shelly M.J., and Garritona, P. (1995). Oviposition in Simulium branchycladum (Diptera: Simuliidae) in Brazil. Entomologist Monthly Magazine 131:171-172.
- Usip, L.P.E., Opara, K.N., Ibanga, E.S and Atting, I.A. (2006). Longitudinal Evaluation of repellant activity of Ocimal gratissim (Labiatae) volatile oil against S. damnosum. Memorias do lastituto Oswaldo Cruz 101(2): 201-205.
- Wilson, M.D., Osei-Atweneboana M.Y., Boakey, D.A., and McCall J.P., (2000). Improved survival and oviposition of Simulium damnosum (Diptera:Simuliidae) in the laboratory. Bulletin of Entomological Research. 90:285-289.
- Wilson M.D., Cheke R.A., Flasse S.P.J., Grist S., Osei-Atweneboana, M.Y., Tetteh-Kmah A., Fiasorgbor G.K., Jolliffe F.R., Boakey D.A., Hougard, J.M., Yameogo L., and Post R.J., (2002). Deforestation and the saptio-temporal distribution Savanna and Forest members of the Simulium damnosum complex in Southern Ghana and Western Togo. Trans. Royal Society for Tropical Medicine and Hygiene. 96:632-639.







