Manuscript accepted on :April 04, 2008
Published online on: --
Plagiarism Check: Yes
Malek Taher Maghsoodlou*, Reza Heydari, Norollah Hazeri, Sayyed Mostafa Habibi-Khorassani, Mahmoud Nassiri, Mohammad Amin Kazemian, Jaber Salehzadeh, Mahbobeh Hajizadeh, Marjan Ghasemzadeh and Elaheh Mosaddegh
Department of Chemistry, The University of Sistan and Balouchestan.
Abstract
Triphenylphosphine reacts with dialkyl acetylenedicarboxylates in the presence of biological active NH heterocyclic compounds, such as (2H) -3-pyridazinone, 1-phenyl-3-pyrazolid- inone, 2,4-thiazolinedione, Rhodanine, 4,5,6,7-tetrahydroindazole and 3-methyl- pyrazole to generate stable phosphorus ylides. These compounds exist in solution as a mixture of two geometrical isomers as a result of restricted rotation around the carbon-carbon partical double bond resulting from conjugation of the ylide moiety with the adjacent carbonyl group.
Keywords
Activated acetylenic esters; NH heterocyclic compounds; Triphenyl- phosphine
Download this article as:| Copy the following to cite this article: Maghsoodlou M. T , Heydari R , Hazeri N , Habibi-Khorassani S. M , Nassiri M , Kazemian M. A , Salehzadeh J , Hajizadeh M, Ghasemzadeh M , Mosaddegh E. Synthesis of Heterocyclic Stable Phosphorus Ylides from Reaction Between Triphenylphosphine and Activated Acetylenic Esters in the Presence of Biological Active NH Heterocyclic Compounds. Biomed Pharmacol J 2008;1(1). |
| Copy the following to cite this URL: Maghsoodlou M. T , Heydari R , Hazeri N , Habibi-Khorassani S. M , Nassiri M , Kazemian M. A , Salehzadeh J , Hajizadeh M, Ghasemzadeh M , Mosaddegh E. Synthesis of Heterocyclic Stable Phosphorus Ylides from Reaction Between Triphenylphosphine and Activated Acetylenic Esters in the Presence of Biological Active NH Heterocyclic Compounds. Biomed Pharmacol J 2008;1(1). Available from: http://biomedpharmajournal.org/?p=204 |
Introduction
Phosphorus-carbon bond formation[1-15] is an active and important research area, as new reactions are continuously being developed for the preparation of organophosphorus compounds such as phosphinates and phosphonates.[16-24] Over the last few years, the quest for the synthetic efficiency has gained remarkable importance, partly due to the need reduce waste.25 Given the increasing industrial, biological and synthetic impact of organophosphorus compounds.[26-30] The successful attack by nucleophilic trivalent phosphorus on a carbon atom is facilitated when the latter is part of, or conjugated with, a carbonyl group, or when it is part of an unsaturated bond otherwise activated.[27-29] There are many studies on the reaction between trivalent phosphorus nucleophiles and α, β–unsaturated carbonyl compounds in the presence of a proton source such as alcohol or phenol.[31,32] Here we wish to report a simple one-pot synthesis of heterocyclic compounds-containing stable phosphorus ylides. Previously, the pyrazole and thiazole moieties and their derivatives have been used commercially as pharmaceuticals, pesticides and dyestuffs.[33] Thus, the reaction of triphenylphosphine 1 with dialkyl acetylenedicarboxylates 2 in the presence of NH heterocyclic compounds 3 such as 3-methylpyrazole and etc were undertaken to generate the corresponding stable heterocyclic phosphorus ylides 4 in excellent yields (see Scheme 1).
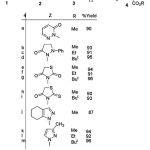 |
Scheme 1
|
Result and discussion
In the current work, stable phosphorus ylides from reaction between triphenylphosphine 1 and dialkyl acetylenedicarboxylates 2 in the presence of N-H acids 3, such as (2H)-3-pyridazinone, 1-phenyl-3-pyrazolidinone, 2,4-thiazolinedi- one, Rhodanine, 4,5,6,7-tetrahydroindazole and 3-methylpyrazole led to 4 in excellent yields (see Scheme 1). The reactions (4a-m) were carried out in ethyl acetate solvent at room temperature and were finished within a few minutes. The 1H and 13C NMR spectrum of the crude product clearly indicated the formation of compounds 4a-m. Any products other than 4a-m could not be detected by NMR spectroscopy. The structures of compounds 4a-m were deduced from their IR, 1H, 13C, 31P NMR spectra, Mass spectrometry and elemental analysis. The mass spectra of compounds 4a-m displayed molecular ion peaks at appropriate values, which were consistent with 1:1:1 adducts of NH heterocyclic compounds, dialkyl acetylenedicarboxylates and triphenylphosphine. Although the presence of the 31P nucleus complicates both the 1H and 13C NMR spectra of 4a, it helps in assignment of the signals by long-range couplings with the 1H and 13C nuclei (see Experimental section). The 1H, 13C, and 31P NMR spectra of ylides 4a-m are consistent with the presence of two isomers. The ylides moieties of these compounds are strongly conjugated with the adjacent carbonyl group and rotation around the partial double bond in (E)-4 and (Z)-4 geometrical isomers is slow on the NMR timescale at ambient temperature (see Scheme 2). As can be seen, only one geometrical isomer was observed for di-tert-butyl derivatives of 4, presumeably, because of the bulky tert-butyl groups. (see Scheme 2).
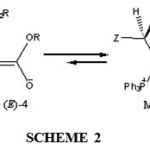 |
Scheme 2
|
On the basis of the well established chemistry of trivalent phosphorus nucleophiles, [3-7] it is reasonable to assume that phosphorus ylide 4 results from the initial addition of triphenylphosphine to the acetylenic esters and subsequent protonation of the 1:1 adduct by the NH heterocyclic compounds to form phosphoranes 4 (see Scheme 3).
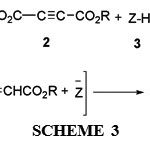 |
Scheme 3
|
The 1H NMR 500 MHz spectra of 4a showed four sharp lines at δ = 3.14, 3.72, 3.61 and 3.66 ppm arising from methoxy protons. Methine protons appeared as two doublet peaks at δ= 5.64 ppm (1H, d, 3JPH=17.0 Hz, P-C-CH) and 5.75 ppm (1H, d, 3JPH=18.9 Hz, P-C-CH) respectively for the E and Z geometrical isomers. The aromatic protons appeared as a multiplet at δ = 6.65–7.90 ppm (18H, m, 3C6H5 and C4H4N2O). The 13C NMR spectrum of 4a displayed 28 distinct resonances in a good agreement with the mixture of two conformational isomers. The 1H and 13C NMR spectra of compounds 4b-m are similar to those of 4a, except for signals from the ester group which appear as characteristic resonance lines with the corresponding chemical shifts. The structural assignments of 4a-m were made on the basis of the 1H and 13C NMR spectra that were supported by their IR spectra. The carbonyl region of the spectra exhibited two distinct absorption bands for each compound related to ester groups of yilde moiety (see Experimental section).
Briefly, we have prepared novel phosphorus ylides using a one-pot reaction between triphenylphosphine and dialkyl acetylenedicarboxylates in the presence of strong NH heterocyclic compounds. The present method carries the advantage that, not only is the reaction performed under neutral conditions, but also the substances can be mixed without any activation or modifications. Heterocyclic compounds-containing stable phosphorus ylides 4a-m may be considered as potentially useful synthetic intermediates. It seems that, the procedure described here may be employed as an acceptable method for the preparation of phosphoranes with variable functionalities.
Experimental
Melting points and IR spectra of all compounds were measured on an Electrothermal 9100 apparatus and a Shimadzu IR-460 spectrometer respectively. Also, the 1H, 13C, and 31P NMR spectra were obtained from a BRUKER DRX-500 AVANCE instrument with CDCl3 as an solvent at 500.1, 125.8, and 202.4 MHz respectively. Elemental analyses for C, H, N were performed using a Heraeus CHN-O-Rapid analyzer. In addition, the mass spectra were recorded on a GCMS-QP 5050A mass spectrometer operating at an ionization potential of 70 eV. (2H)-3-pyridazinone, 1-phenyl-3-pyrazolidinone, 2,4-thiazolinedione, Rhodanine, 4,5,6,7-tetrahydroindazole, 3-methylpyrazole dialkyl acetylenedicarboxylates and triphenlphosphine, were purchsed from Fluka, (Buchs, Switzerland) and used without further purifications.
General Procedures (Exemplified by 4a)
Preparation of dimethyl 2-( 3-pyridazinone-2-yl)-3-(triphenylphosphanylidene) butanedioate (4a).
To a magnetically stirred solution of triphenylphosphine (0.26 g or 1 mmol) and (2H)-3-pyridazinone (0.96 g or 1 mmol) in 10 mL of dry ethyl acetate was added, dropwise, a mixture of dimethyl acetylenedicarboxylate (0.14g or 1 mmol) in 5 mL of dry ethyl acetate over 10 min. After a few minutes stirring at room temperature, the product was filtered and washed with cold diethyl ether (3 × 5 mL).
White powder, m.p 93-95°C, 0.46 g, yield 90%, IR (vmax, cm-1): 1733 and 1664 (C=O). 500 (M+,7), 438 (M-2OMe, 37), 382 (M-2CO2Me 46), 262 (PPh3, 80), 183 (PPh2, 48), 108 (PPh, 43), 77 (Ph, 35). Anal. calcd. for C28H25N2O5P (500): C, 67.17; H, 5.04; N, 5.60 %. Found: C, 67.21; H, 4.95; N, 5.67 %.
Major isomer Z-4a (60%): 1H NMR (500.1 MHz, CDCl3): δ 3.14 and 3.72 (6H, 2s, 2OCH3), 5.64 (1H, d, 3JPH = 17.0 Hz, P=C-CH), 6.65-7.90 (18Haro, m, 3C6H5 and C4H3N2O). 13C NMR (125.8 MHz, CDCl3): δ 42.75 (d, 1JPC = 126.8 Hz, P=C), 49.34 and 52.30 (2s, 2OCH3), 63.72 (d, 2JPC=16.0 Hz, P-C-CH), 108.89 (1C, C4H3N2O), 126.37 (d, 1JPC = 92.0 Hz, Cipso), 127.84 (d, 3JPC = 11.9 Hz, Cmeta), 132.19 (d, 4JPC = 2.1 Hz, Cpara), 133.57 (1C, C4H3N2O), 133.28 (d, 2JPC = 9.0 Hz, Cortho), 147.55 and 162.13 (2C, C4H3N2O), 165.42 (d, 3JPC = 15.7Hz, C=O), 171.57 (d, 2JPC = 13.5 Hz, P-C=C). 31P NMR (202.4 MHz, CDCl3): δ 23.12 (Ph3P+-C).
Minor isomer E-4a (40%): 1H NMR (500.1 MHz, CDCl3): δ 3.61 and 3.66 (6H, 2s, 2OCH3), 5.75 (1H, d, 3JPH = 18.9 Hz, P=C-CH), 6.65-7.90 (18Haro, m, 3C6H5 and C4H3N2O). 13C NMR (125.8 MHz, CDCl3): δ 42.86 (d, 1JPC = 136.0 Hz, P=C), 50.32 and 52.32 (2s, 2OCH3), 63.57 (d, 2JPC = 15.8 Hz, P-C-CH), 108.93 (1C, C4H3N2O), 125.67 (d, 1JPC = 92.1 Hz, Cipso), 128.27 (d, 3JPC = 12.0 Hz, Cmeta), 132.23 (d, 4JPC = 2.2 Hz, Cpara), 134.13 (1C, C4H3N2O), 133.59 (d, 2JPC = 9.8 Hz, Cortho), 147.63 and 163.14 (2C, C4H3N2O), 165.72 (d, 3JPC =14.8 Hz, C=O), 169.83 (d, 2JPC = 12.5 Hz, P-C=C). 31P NMR (202.4 MHz, CDCl3): δ 22.97 (Ph3P+-C ).
dimethyl-2-(1-phenyl-3-pyrazolidinone-2-yl)-3-(triphenylphosphanylidene) butanedioate (4b).
white powder, m.p 166-168°C, 0.53g, yield 93%, IR (υmax, cm-1) 1753 and 1676 (C=O). MS (m/z, %): 446 (M-2CO2Me, 17), 304 (M-PPh and Ph, 38), 262 (PPh3, 76), 183 (PPh2, 36), 108 (PPh, 43), 77 (Ph, 14). Anal. calcd. for C33H31N2O5P (566): C, 70.12; H,5.56; N, 5.02%. Found: C, 69.96; H, 5.48; N, 4.95%.
Major isomer Z-4b (68%): 1H NMR (500.1 MHz, CDCl3): δ 2.43(2H, m, H2C-CO), 3.20(3H, s, OCH3), 3.55(2H, m, H2C-N), 3.81 (3H, s, OCH3), 4.97 (1Hbro, P-C-CH), 6.99–7.71 (20Harom, m, 4C6H5). 13C NMR (125.8 MHz, CDCl3): δ 29.65 and 30.03 (2C, C9H9N2O), 38.18 (d, 1JPC = 128.8 Hz, P=C), 49.68 and 52.08 (2s, 2OCH3), 59.79 (d, 2JPC = 16.2 Hz, P-C-CH), 116.56, 119.76, 123.03, 126.17 and 126.91 (5C, C9H9N2O), 126.54 (d, 1JPC = 92.9 Hz, Cipso), 128.50 (d, 3JPC = 12.3 Hz, Cmeta), 131.85 (Cpara), 132.07 (1C, C9H9N2O), 133.73 (d, 2JPC = 9.8 Hz, Cortho), 170.43 (d, 3JPC = 16.8 Hz, C=O), 171.89 (d, 2JPC = 17.6 Hz, P-C=C), 176.59 (1C, C9H9N2O). 31P NMR (202.4 MHz, CDCl3): δ 23.56 (Ph3P+-C).
Minor isomer E-4b (32%): 1H NMR (500.1 MHz, CDCl3): δ 2.52(2H, m, H2C-CO), 3.44(2H, m, H2C-N), 3.74 and 3.79 (6H, 2s, 2OCH3), 4.93 (1Hbro, P-C-CH), 6.99–7.71 (20Harom, m, 4C6H5). 13C NMR (125.8 MHz, CDCl3): δ 29.54 and 29.96 (2C, C9H9N2O), 39.76 (d, 1JPC = 139.4 Hz, P=C), 52.01 and 52.27 (2s, 2OCH3), 60.20 (d, 2JPC = 15.2 Hz, P-C-CH), 116.52, 119.80, 123.07, 126.23 and 126.86 (5C, C9H9N2O), 126.17 (d, 1JPC = 92.0 Hz, Cipso), 128.60 (d, 3JPC = 11.8 Hz, Cmeta), 131.69 (Cpara), 132.15 (1C, C9H9N2O), 133.64 (d, 2JPC = 10.0 Hz, Cortho), 172.02 (d, 3JPC = 14.3 Hz, C=O), 171.74 (d, 2JPC = 15.4 Hz, P-C=C), 177.11 (1C, C9H9N2O). 31P NMR (202.4 MHz, CDCl3): δ 24.90 (Ph3P+-C ).
Diethyl-2-(1-phenyl-3-pyrazolidinone-2-yl)-3-(triphenylphosphanylidene) butanedioate (4c).
White powder, m.p 161-163°C, 0.54g, yield 91%, IR (υmax, cm-1) 1748 and 1662 (C=O). MS (m/z, %): 502 (M-2OEt , 50), 434 (M-PPh and OEt, 28), 330 (M-PPh3, 7), 262 (PPh3, 85), 183 (PPh2, 83), 108 (PPh, 31). Anal. calcd. for C35H35N2O5P (594): C, 71.35; H, 6.05; N, 4.64%. Found: C, 70.71; H, 5.95; N, 4.71%.
Major isomer Z-4c (66%): 1H NMR (500.1 MHz, CDCl3): δ 0.36 and 1.27 (6H, 2t, 3JHH = 7.0 Hz, 2OCH2CH3), 2.41(2H, m, H2C-CO), 3.48 and 3.54 (4H, 2m, 2ABX3 system, 2OCH2CH3), 3.59(2H, m, H2C-N), 4.26 (1Hbro, P-C-CH), 7.12-7.73 (20Harom, m, 4C6H5). 13C NMR (125.8 MHz, CDCl3): δ 13.96 and 14.13 (2s, 2OCH2CH3), 29.61 and 30.09 (2C, C9H9N2O), 37.89 (d, 1JPC = 128.9 Hz, P=C), 57.45 and 58.05 ( 2S, 2OCH2CH3), 59.98 (d, 2JPC = 14.8 Hz, P-C-CH), 116.59, 119.80, 122.96, 126.35 and 127.08 (5C, C9H9N2O), 126.27 (d, 1JPC = 92.0 Hz, Cipso), 128.35 (d, 3Jpc = 12.1 Hz, Cmeta), 131.63 (Cpara), 132.07 (1C, C9H9N2O), 133.88 (Cortho), 170.69 (d, 3JPC = 17.7 Hz, C=O), 171.19 (d, . 2JPC = 16.3 Hz, P-C=C), 176.99 (1C, C9H9N2O).31P NMR (202.4 MHz, CDCl3): δ 23.35 (Ph3P+-C)
Minor isomer E-4c (34%): 1H NMR (500.1 MHz, CDCl3): δ 1.09 and 1.32 (6H, 2t, 3JHH = 7.2 Hz , 2OCH2CH3), 2.53(2H, m, H2C-CO), 3.41(2H, m, H2C-N), 3.91 and 4.06 (4H, 2m, 2ABX3 system, 2OCH2CH3), 4.31 (1Hbro, P-C-CH), 7.12-7.73 (20Harom, m, 4C6H5). 13C NMR (125.8 MHz, CDCl3): δ 14.13 and 14.73 (2s, 2OCH2CH3), 29,53 and 30.01 (2C, C9H9N2O), 39.69 (d, 1JPC =133.3 Hz, P=C), 58.01 and 58.39 (2s, 2OCH2CH3), 60.41 (d, 2JPC = 15.1 Hz, P-C-CH), 116.51, 120.05, 123.20, 126.42 and 127.15 (5C, C9H9N2O), 126.72 (d, 1JPC=91.8 Hz, Cipso), 128.54 (d, 3Jpc = 12.0 Hz, Cmeta), 129.19 (1C, C9H9N2O), 131.76 (Cpara), 132.14 (1C, C9H9N2O), 133.88 (Cortho), 169.98 (d, 3JPC =16.4 Hz, C=O), 170.12 (d, 2JPC = 15.9 Hz, P-C=C),177.26 (1C, C9H9N2O). 31P NMR (202.4 MHz, CDCl3): δ 23.52 (Ph3P+-C ).
Di-tert-buthyl-2-(1-phenyl-3-pyrazolidinone-2-yl)-3-(triphenylphosphanylidene) butanedioate (4d).
White crystals, m.p. 176-178 ºC, 0.62g, yield 96%; IR (νmax, cm-1): 1746 and 1671 (C=O). MS (m/z, %): 650 (M, 3), 386 (M-PPh3, 38), 262 (PPh3, 79), 183 (PPh2, 43), 108 (PPh, 51). Anal. calcd. for C39H43N2O5P (650): C, 71.08; H, 6.72; N, 4.28%. Found: C, 71.56; H, 6.68; N, 4.31%.
Major rotamer Z-4d 1H NMR (500.1 MHz, CDCl3), δH 0.80 and 1.49 (18H, 2s, 2OCMe3), 2.50(2H, m, H2C-CO), 3.46(2H, m, H2C-N), 4.67 (1H, d, 3JPH = 17.4 Hz, P-C-CH), 7.03-7.69 (20Harom, m, 4C6H5). 13C NMR (125.8 MHz, CDCl3), δ 28.16 and 28.43 (2OCMe3), 29.76 and 30.12 (2C, C9H9N2O), 37.63 (d, 1JPC = 127.2 Hz, P=C), 60.79 (d, 2JPC =14.9 Hz, P-C-CH), 80.60 and 80.83 (2s, 2OCMe3), 116.61, 119.36, 121.17, 122.75 and 123.66 (5C, C9H9N2O), 127.18 (d, 1JPC = 91.2 Hz, Cipso), 128.31 (d, 3JPC = 11.8 Hz, Cmeta), 131.56 (Cpara), 132.22 (1C, C9H9N2O), 133.82 (d, 2JPC = 9.4 Hz, Cortho), 169.98(d, 3JPC = 11.6 Hz, C=O), 171.03 (d, 2JPC = 11.7 Hz, P-C=C), 177.74 (1C, C9H9N2O). 31P NMR (202.4 MHz, CDCl3): δP 23.29 (Ph3P+-C).
Dimethyl -2-( 2,4-thiazolidinedione-2-yl)-3-(triphenylphosphanylidene) butaned- ioate (4e).
White crystals, m.p. 95-97 ºC, 0.48g, yield 94%; IR (νmax, cm-1): 1739 and 1685, 1662 and 1610 (C=O); MS, (m/z, %): 509 (M+, 1), 334 (M-C3H2O2NS and CO2Me, 29), 278 (M-3Ph, 69), 262 (PPh3, 83), 183 (PPh2, 86), 108 (PPh, 36). Anal. calcd. for C27H24NO6PS (521): C, 62.17; H, 4.64; N, 2.69 %. Found: C, 62.28; H, 4.60; N, 2.65%.
Major rotamer Z-4e (63%): 1H NMR (500.1 MHz, CDCl3), δH 3.11 and 3.70 (6H, 2s, 2OCH3), 3.75 (2H, dd, 2JHH = 16.0, S-CH2), 4.78 (1H, d, 3JPH = 15.0 Hz, P-C-CH), 7.45-7.70 (15Harom, m, 3C6H5); 13C NMR (125.8 MHz, CDCl3), δC 32.98 (1C, S-CH2), 36.33 (d, 1JPC = 131.1 Hz, P=C), 49.08 and 52.88 (2OCH3), 58.96 (d, 2JPC = 18.3 Hz, P-C-CH), 126.71 (d, 1JPC = 91.7 Hz, Cipso), 128.87 (d, 3JPC = 10.7 Hz, Cmeta), 132.19 (Cpara), 133.60 (d, 2JPC = 9.8 Hz, Cortho), 170.29 (d, 3JPC = 12.3 Hz, C=O), 170.59 (d, 2JPC = 17.6 Hz, P-C=C), 171.39 (1C, CH2–CO), 172.01 (1C, S-CO); 31P NMR (202.4 MHz, CDCl3): δP 22.68 (Ph3P+-C).
Minor rotamer E-4e (37%): 1H NMR (500.1 MHz, CDCl3), δH 3.58 and 3.69 (6H, 2s, 2OCH3), 3.73 (2H, dd, 2JHH = 16.1, S-CH2), 4.81 (1H, d, 3JPH = 15.9 Hz, P-C-CH), 7.45-7.70 (15Harom, m, 3C6H5); 13C NMR (125.8 MHz, CDCl3), δC 32.74 (1C, S-CH2), 38.15 (d, 1JPC = 137.0 Hz, P=C), 50.53 and 52.64 (2 OCH3), 58.54 (d, 2JPC = 17.6 Hz, P-C-CH), 126.05 (d, 1JPC = 91.7 Hz, Cipso), 128.51 (d, 3JPC = 12.1 Hz, Cmeta), 132.16 (Cpara), 133.64 (d, 2JPC = 9.7 Hz, Cortho), 170.46 (d, 3JPC = 17.5 Hz, C=O), 171.01 (d, 2JPC = 18.7 Hz, P-C=C), 171.27 (1C, CH2–CO), 172.05 (1C, S-CO); 31P NMR (202.4 MHz, CDCl3): δP 22.52 (Ph3P+-C).
Diethyl -2-(2,4-thiazolidinedione-2-yl)- 3-(triphenylphosphanylidene) butaned- ioate (4f).
White crystals, m.p. 134-136 ºC, 0.49g, yield 91%; IR (νmax, cm-1): 1740, 1715, 1680 and 1636 (C=O). MS, (m/z, %): 391 (M-2CO2Et, 53), 275 (M-PPh3, 74), 262 (PPh3, 74), 183 (PPh2, 75), 108 (PPh, 37). Anal. calcd. for C29H28NO6PS (549): C, 63.36; H, 5.14; N, 2.55 %. Found: C, 63.30; H, 5.21; N, 2.49%.
Major rotamer Z-4f (71%): 1H NMR (500.1 MHz, CDCl3), δH 0.44 and 1.26 (6H, 2t, 3JHH = 6.8 Hz 2OCH2CH3), 3.68 (2H, dd, 2JHH = 16.5 Hz, S-CH2), 3.76 and 4.14 (4H, 2m, 2ABX3 systhem 2OCH2CH3), 4.79 (1H, d, 3JPH = 15.9 Hz, P-C-CH), 7.48-7.73 (15Harom, m, 3C6H5); 13C NMR (125.8 MHz, CDCl3), δC 14.12 and 14.33 (2s, 2OCH2CH3), 32.97 (1C, S-CH2), 36.12 (d, 1JPC = 130.5 Hz, P=C), 57.62 and 59.14. ( 2S, 2OCH2CH3), 59.78 (d, 2JPC = 18.2 Hz, P-C-CH), 126.42 (d, 1JPC = 91.8 Hz, Cipso), 128.73 (d, 3JPC = 11.2 Hz, Cmeta), 132.09 (Cpara), 133.64 (Cortho), 168.39 (d, 3JPC = 12.4 Hz, C=O), 169.77 (d, 2JPC = 11.6 Hz, P-C=C), 170.60 (1C, CH2–CO), 170.67 (1C, S-CO); 31P NMR (202.4 MHz, CDCl3): δP 22.66 (Ph3P+-C).
Minor rotamer E-4f (29%): 1H NMR (500.1 MHz, CDCl3), δH 1.18 and 1.30 (6H, 2t, 3JHH = 7.2 Hz, 2OCH2CH3), 3.74 (2H, dd, 2JHH = 16.3 Hz, S-CH2), 4.04 and 4.24 (4H, 2m, 2ABX3systhem 2OCH2CH3), 4.77 (1H, d, 3JPH = 18.0 Hz, P-C-CH), 7.48-7.73 (15Harom, m, 3C6H5); 13C NMR (125.8 MHz, CDCl3), δC 13.88 and 13.91 (2s, 2OCH2CH3), 32.86 (1C, S-CH2), 38.04 (d, 1JPC = 138.8 Hz, P=C), 57.67 and 59.36 (2s, 2OCH2CH3), 59.94 (d, 2JPC = 17.0 Hz, P-C-CH), 126.37 (d, 1JPC = 92.1 Hz, Cipso), 128.50 (d, 3JPC = 12.1 Hz, Cmeta), 132.14 (Cpara), 133.60 (Cortho), 168.48 (d, 3JPC = 12.7 Hz, C=O), 169.67 (d, 2JPC = 14.8 Hz, P-C=C), 170.45 (1C, CH2–CO), 170.85 (1C, S-CO); 31P NMR (202.4 MHz, CDCl3): δP 22.71 (Ph3P+-C).
Di-tert-buthyl-2-(2,4-thiazolidinedione-1-yl)-3-(triphenylphosphanylidene) butanedioate (4g).
White crystals, m.p. 133-135 ºC, 0.58g, yield 96%; IR (νmax, cm-1): 1737, 1729, 1665 and 1639 (C=O). ). MS, (m/z, %): 343 (M-PPh3, 9), 276 (M-PPh2 and 2OCMe3,38 ), 262 (PPh3, 75), 227 (M-PPh3 and C3H2O2NS, 6), 183 (PPh2, 63), 108 (PPh, 26). Anal. calcd. for C33H36NO6PS (605): C, 65.42; H, 5.99; N, 2.31 %. Found: C, 65.51; H, 6.05; N, 2.35%.
Major rotamer Z-4g : 1H NMR (500.1 MHz, CDCl3), δH 0.94 and 1.54 (18H, 2s, 2OCMe3), 3.72 (2H, dd, 2JHH = 16.9 Hz, S-CH2), 4.62 (1H, d, 3JPH = 17.3 Hz, P-C-CH), 7.48-7.75 (15Harom, m, 3C6H5); 13C NMR (125.8 MHz, CDCl3), δC 28.32 and 28.37 (2OCMe3), 32.82 (1C, S-CH2), 36.13 (d, 1JPC = 130.8 Hz, P=C), 60.54 (d, 2JPC = 17.9 Hz, P-C-CH), 77.17 and 81.26 (2s, 2OCMe3), 127.51 (d, 1JPC = 91.7 Hz, Cipso), 128.64 (d, 3JPC = 11.7 Hz, Cmeta), 132.01(Cpara), 133.64 (d, 2JPC = 9.8 Hz, Cortho), 167.68 (d, 3JPC = 12.6 Hz, C=O), 168.66 (d, 2JPC=13.3 Hz, P-C=C), 169.82 (1C, CH2–CO), 170.74 (1C, S-CO); 31P NMR (202.4 MHz, CDCl3): δP 23.15 (Ph3P+-C).
Dimethyl 2-(rhodanine-1-yl)-3-(triphenylphosphanylidene) butanedioate (4h).
white crystals, m.p 108-110 °C, 0.48 g , yield 90 % . IR (KBr) (vmax, cm-1): 1722, 1747 and 1623 (C=O). MS, (m/z, %): 537 (M+, 6), 475 (M-2OCH3, 52), 419 (M-2CO2CH3, 36), 262 (PPh3, 85), 183 (PPh2, 61), 120 (C3H2NOS2, 29), 108 (PPh, 44), Anal. calcd. for C27H24NO5PS2 (537): C, 60.31; H, 4.50; N, 2.67 %. Found: C, 60.25; H, 4.58; N, 2.75%.
Major isomer Z-4h (66%): 1H NMR (500.1 MHz, CDCl3): δ 3.07 and 3.69 (6H, 2s, 2OCH3), 3.83 (2H, s, S-CH2), 5.39 (1H, d, 3JPH = 14.4 Hz, P=C-CH), 7.47-7.72 (15Haro, m, 3C6H5). 13C NMR (125.8 MHz, CDCl3): δ 33.90 (1C, S-CH2), 43.50 (d, 1JPC = 126.2 Hz, P=C), 49.34 and 52.30 (2s, 2OCH3), 63.25 (d, 2JPC = 15.7 Hz, P-C-CH), 126.30 (d, 1JPC = 92.1 Hz, Cipso), 128.57 (d, 3JPC = 12.2 Hz, Cmeta), 132.01 (d, 4JPC = 2.2 Hz, Cpara), 133.47 (d, 2JPC = 8.9 Hz, Cortho), 165.4 (C=O), 170.60 (1C, CH2–CO), 170.69 (1C, S-CS); 171.59 (d, 2JPC = 12.5 Hz, P-C=C). 31P NMR (202.4 MHz, CDCl3): δ 22.50 (Ph3P+-C).
Minor isomer E-4h. (34%): 1H NMR (500.1 MHz, CDCl3): δ 3.60 and 3.71 (6H, 2s, 2OCH3), 3.81 (2H, s, S-CH2), 5.51 (1H, d, 3JPH = 17.4 Hz, P=C-CH), 7.47-7.72 (15Haro, m, 3C6H5). 13C NMR (125.8 MHz, CDCl3): δ 33.97 (1C, S-CH2), 45.09 (d, 1JPC =137.3 Hz, P=C), 51.36 and 52.47 (2s, 2OCH3), 63.78 (d, 2JPC = 15.9 Hz, P-C-CH), 125.86 (d, 1JPC=92.0 Hz, Cipso), 128.90 (d, 3JPC = 12.0 Hz, Cmeta), 132.29 (d, 4JPC = 2.4 Hz, Cpara), 133.59 (d, 2JPC = 9.8 Hz, Cortho), 165.7 (C=O), 169.83 (d, 2JPC = 12.3 Hz, P-C=C). 170.96 (1C, CH2–CO), 171.16 (1C, S-CS); 31P NMR (202.4 MHz, CDCl3): δ 22.42 (Ph3P+-C ).
Di-tert-butyl 2-(rhodanine-1-yl)-3-(triphenylphosphanylidene) butanedioate (4i).
White crystals, m.p 157-159 ºC, 0.58 g, yield 93 % . IR (vmax, cm-1): 1726, 1638 (C=O). MS, (m/z, %): 621 (M+, 5), 507 (M-2CMe3, 34), 475 (M+-2OCMe3, 49), 262 (PPh3, 85), 183 (PPh2, 60), 108 (PPh, 51), Anal. calcd. for C33H36NO5PS2 (621): C, 63.74; H, 5.84; N, 2.30 %. Found: C, 63.80; H, 5.91; N, 2.26%.
Major isomer Z-4i (87%): 1H NMR (500.1 MHz, CDCl3), δ 1.28 and 1.39 (18H, 2s, 2CMe3), 3.73 (2H, d, S-CH2), 4.63 (1H, d, 3JPH = 16.6 Hz, P-C-CH), 7.27-7.73 (15Haro, m, 3C6H5). 13C NMR (125.8 MHz, CDCl3), δ 28.25 and 28.48 (2s, 2CMe3), 34.12 (1C, S-CH2), 40.92 (d, 1JPC =126.9 Hz, P=C), 64.33 (d, 2JPC = 14.7 Hz, P-C-CH), 77.92 and 80.43 (2s, 2OCMe3), 127.84 (d, 1JPC = 91. 6 Hz, Cipso), 128.31 (d, 3JPC = 12.2 Hz, Cmeta), 131.70 (Cpara), 133.99 (d, 2JPC = 9.7 Hz, Cortho), 167.85 (d, 3JPC =12.2 Hz, C=O), 170.06 (d, 2JPC =13.8 Hz, P-C=C). 171.63 (1C, CH2–CO), 171.97 (1C, S-CS); 31P NMR (202.4 MHz, CDCl3): δ 22.09 (Ph3P+-C).
Dimetyl-2-( 4,5,6,7-tetrahydroindazole-1-yl)-3-(triphenylphosphanylidene) butanedioate (4j).
white crystals, m.p 154-156°C, 0.45 g, yield 87 % . IR(KBr) (vmax, cm-1): 1753 and 1636 (2C=O). MS (m/z, %): 526 (M+, 6), 501 (M+-CO2Me, 7), 467 (M+-CO2CH3,100), 405(M+-C7H9N2,65), 262 (PPh3, 80), 183 (PPh2, 100), 108 (PPh, 40), 77 (Ph, 15), 59 (CO2Me, 7). Anal. calcd. for C31H31N2O5P (526): C, 70.69; H, 5.94; N, 5.32 %. Found: C, 70.63; H, 5.98; N, 5.38%.
Major isomer Z-4j (52%): 1H NMR (500.1 MHz, CDCl3): δ 1.73 and 2.68 (8H, m, 4CH2), 3.14 and 3.72 (6H, 2s, 2OCH3), 4.90 (1H, d, 3JPH = 16.7 Hz, P=C-CH), 7.45-7.72 (16Haro, m, 3C6H5 and C7H9N2). 13C NMR (125.8 MHz, CDCl3): 20.87, 23.23 and 23.54 (4 CH2), 43.72 (d, 1JPC = 128.2 Hz, P=C), 49.26 and 52.35 (2s, 2OCH3), 64.54 (d, 2JPC = 15.0 Hz, P=C-CH), 115.45 (1C, N-C=C), 126.44 (d, 1JPC = 92.9 Hz, Cipso), 128.49 (d, 3JPC = 12.1 Hz, Cmeta), 131.92 (Cpara), 133.40 (1C, N-N=C), 133.64 (d, 2JPC = 9.9 Hz, Cortho), 146.90 (1C, N-C=C), 165.37 (C=O), 172.59 (d, 2JPC = 13.0 Hz, P-C=C). 31P NMR (202.4 MHz, CDCl3): δ 24.18 (Ph3P+-C).
Minor isomer E-4j (48%): 1H NMR (500.1 MHz, CDCl3): δ 1.73 and 2.68 (8H, m, 4CH2), 3.61 and 3.72 (6H, 2s, 2OCH3), 4.93 (1H, d, 3JPH = 18.3 Hz, P=C-CH), 7.45-7.72 (16H, m, 3C6H5 and C7H9N2). 13C NMR (125.8 MHz, CDCl3): 21.94, 22.90 and 23.63 (4 CH2), 43.94 (d, 1JPC = 133.8 Hz, P=C), 52.22 and 52.53 (2s, 2OCH3), 63.93 (d, 2JPC = 16.3 Hz, P=C-CH), 115.13 (1C, N-C=C), 125.93 (d, 1JPC = 82.4 Hz, Cipso), 128.81 (d, 3JPC = 12.3 Hz, Cmeta), 131.92 (Cpara), 133.19 (1C, N-N=C), 133.64 (d, 2JPC = 9.9 Hz, Cortho), 147.43 (1C, N-C=C), 165.68 (C=O), 169.78 (d, 2JPC = 12.1 Hz, P-C=C), 31P NMR (202.4 MHz, CDCl3): δ 23.06 (Ph3P+-C ).
Dimethyl 2-(3-methylpyrazole-1-yl)-3-(triphenylphosphanylidene) butanedioate (4k).
White powder, m.p 183-144°C, 0.46 g, yield 94%, IR (vmax, cm-1): 1755 and 1638 (C=O). MS (m/z, %): 486 (M+, 9), 423 (M+-CO2Me, 100), 405 (M+-C4H5N2, 22), 262 (PPh3, 35), 183 (PPh2, 50), 108 (PPh, 20), 77 (Ph, 8). Anal. calcd. for C28H27N2O4P (486): C, 69.11; H, 5.60; N, 5.76 %. Found: C, 68.97; H, 5.65; N, 5.80 %.
Major isomer Z-4k (55%): 1H NMR (500.1 MHz, CDCl3): δ 2.17 (3H, s, CH3), 3.20 and 3.72 (6H, 2s, 2OCH3), 4.91 (1H, d, 3JPH = 16.6 Hz, P=C-CH), 7.28-7.95 (17Haro, m, 3C6H5 and C4H5N2). 13C NMR (125.8 MHz, CDCl3): δ 13.52 (s, CH3), 43.76 (d, 1JPC = 127.7 Hz, P=C), 49.34 and 52.30 (2s, 2OCH3), 64.52 (d, 2JPC = 15.9 Hz, P-C-CH), 104.97 (1C, C4H5N2), 126.30 (d, 1JPC = 92.1 Hz, Cipso), 128.57 (d, 3JPC = 12.2 Hz, Cmeta), 132.01 (d, 4JPC = 2.2 Hz, Cpara), 132.13 (1C, C4H4N2), 133.47 (d, 2JPC = 8.9 Hz, Cortho), 146.50 (N-N=C), 165.4 (C=O), 172.50 (d, 2JPC =12.6 Hz, P-C=C). 31P NMR (202.4 MHz, CDCl3): δ 24.08 (Ph3P+-C).
Minor isomer E-4k (45%): 1H NMR (500.1 MHz, CDCl3): δ 2.20 (3H, s, CH3), 3.61 and 3.71(6H, 2s, 2OCH3), 4.95 (1H, d, 3JPH = 18.2 Hz, P=C-CH), 7.28-7.95 (17Haro, m, 3C6H5 and C4H5N2). 13C NMR (125.8 MHz, CDCl3): δ 11,98 (s, CH3), 44.04 (d, 1JPC = 138.0 Hz, P=C), 50.32 and 52.32 (2s, 2OCH3), 63.57 (d, 2JPC = 15.8 Hz, P-C-CH), 104.77 (1C, C4H5N2), 125.67 (d, 1JPC = 92.2 Hz, Cipso), 128.84 (d, 3JPC = 12.1 Hz, Cmeta), 132.13 (1C, C4H5N2), 132.29 (d, 4JPC = 2.4 Hz, Cpara), 133.59 (d, 2JPC = 9.8 Hz, Cortho), 147.00 (N-N=C), 165.7 (C=O), 169.83 (d, 2JPC = 12.3 Hz, P-C=C). 31P NMR (202.4 MHz, CDCl3): δ 24.84 (Ph3P+-C ).
Diethyl 2-(3-methylpyrazole-1-yl)-3-(triphenylphosphanylidene) butanedioate (4l).
white crystals, m.p 147-149°C, 0.47 g , yield 92% . IR(KBr) (vmax, cm-1): 1757 and 1635 (C=O). MS (m/z, %): 514 (M+, 3), 441 (M+-CO2Et, 100), 262 (PPh3, 42), 183 (PPh2, 44), 108 (PPh, 21), 77 (Ph, 8). Anal. calcd. for C30H31N2O4P (514): C, 70.00; H, 6.08; N, 5.44 %. Found: C, 70.38; H, 6.13; N, 5.49 %.
Major isomer Z-4l (63%): 1H NMR (500.1 MHz, CDCl3): δ 0.48 and 1.23 (6H, 2t, 3JHH = 7.1Hz, 2OCH2CH3), 2.17 (3H, s, CH3), 3.75 and 4.10 (4H, 2m, 2ABX3 system, 2OCH2CH3), 4.90 (1H, d, 3JPH = 16.8 Hz, P-C-CH), 7.20-7.97 (17Haro, m, 3C6H5 and C4H5N2). 13C NMR (125.8 MHz, CDCl3): 13.56 (s, CH3) 13.56 and 14.21 (2OCH2CH3), 43.40 (d, 1JPC = 127.6 Hz, P=C), 57.75 and 61.09 (2s, 2OCH2CH3), 64.53 (d, 2JPC = 16.6 Hz, P-C-CH), 104.80 (1C, C4H5N2), 126.62 (d, 1JPC = 92.1 Hz, Cipso), 128.70 (d, 3JPC = 12.1 Hz, Cmeta), 132.21 (Cpara), 133.61 (d, 2JPC = 9.8 Hz, Cortho), 133.67 (1C, C4H5N2), 146.13 (1C, N-N=C), 169.21 (d, 3JPC = 12.8 Hz,C=O), 171.68 (d, 2JPC = 12.3 Hz, P-C=C). 31P NMR (202.4 MHz, CDCl3): δ 24.09 (Ph3P+-C).
Minor isomer E-4l (37%): 1H NMR (500.1 MHz, CDCl3): δ 1.18 and 1.30 (6H, 2t, 3JHH=7.1 Hz, 2OCH2CH3), 2.20 (3H, s, CH3), 4.75 and 4.20 (4H, 2m, 2ABX3system, 2OCH2CH3), 4.91 (1H, d, 3JPH = 18.1 Hz, P-C-CH), 7.20-7.97 (17Haro, m, 3C6H5 and C4H5N2). 13C NMR (125.8 MHz, CDCl3): 13.11 (s, CH3), 13.99 and 14.84 (2OCH2CH3), 43.85 (d, 1JPC = 135.6 Hz, P=C), 58.39 and 61.09 (2s, 2OCH2CH3), 63.86 (d, 2JPC = 16.3 Hz, P-C-CH), 104.64 (1C, C4H5N2), 125.96 (d, 1JPC = 92.7 Hz, Cipso), 128.70 (d, 3JPC = 12.1 Hz, Cmeta), 132.21 (Cpara), 133.61 (d, 2JPC = 9.8 Hz, Cortho), 133.67 (1C, C4H5N2), 146.61 (1C, N-N=C), 169.99 (d, 3JPC = 12.8 Hz,C=O), 171.53 (d, 2JPC = 10.6 Hz, P-C=C). 31P NMR (202.4 MHz, CDCl3): δ 24. 95 (Ph3P+-C).
Di-tert-butyl 2-(3-methylpyrazole-1-yl)-3-(triphenylphosphanylidene) butanedioate (4m).
White crystals, m.p 175-177 ºC, 0.55 g, yield 96% . IR (vmax, cm-1): 1749, 1642 (C=O). MS, (m/z, %): 570 (M+, 10), 469 (M+-CO2CMe3, 65), 287 (M+– PPh3-C-CH,85), 262 (PPh3, 50), 183 (PPh2, 55), 108 (PPh, 23), 77 (Ph, 5), 57 (CMe3, 33). Anal. calcd. for C34H39N2O4P (570): C, 71.54; H, 6.89; N, 4.91 %. Found: C, 71.60; H, 6.92; N, 4.89 %.
Major isomer Z-4m (57%): 1H NMR (500.1 MHz, CDCl3), δ 0.95 and 1.50 (18H, 2s, 2CMe3), 2.15 (3H, s, CH3), 4.96 (1H, d, 3JPH = 17.3 Hz, P-C-CH), 6.60-8.00 (17Haro, m, 3C6H5 and C4H5N2). 13C NMR (125.8 MHz, CDCl3), δ 13.56 (s, CH3), 28.18 and 28.37 (2s, 2CMe3), 43.21 (d, 1JPC = 127.1 Hz, P=C), 65.24 (d, 2JPC = 16.74 Hz, P-C-CH), 77.89 and 80.54 (2s, 2OCMe3), 104.68 (1C, C4H5N2), 127.4 (d, 1JPC = 92.0 Hz, Cipso), 128.51 (d, 3JPC = 12.3 Hz, Cmeta), 132.00 (Cpara), 133.71 (d, 2JPC = 9.6 Hz, Cortho), 145.85 (1C, C4H5N2), 168.65 (d, 3JPC = 12.2 Hz, C=O), 170.39 (d, 2JPC = 13.3 Hz, P-C=C). 31P NMR (202.4 MHz, CDCl3): δ 24.91 (Ph3P+-C).
Minor isomer E-4m (43%): 1H NMR (500.1 MHz, CDCl3), δ 1.46 and 1.48 (18H, 2s, 2CMe3), 2.18 (3H, s, CH3), 4.99 (1H, d, 3JPH = 16.9 Hz, P-C-CH), 6.60-8.00 (17Haro, m, 3C6H5 and C4H5N2). 13C NMR (125.8 MHz, CDCl3), δ 11.01 (s, CH3), 28.12 and 28.45 (2s, 2CMe3), 43.55 (d, 1JPC = 138.1 Hz, P=C), 64.20 (d, 2JPC =15.08 Hz, P-C-CH), 77.93 and 80.42 (2s, 2OCMe3), 104.39 (1C, C4H5N2), 127.27 (d, 1JPC = 92.1 Hz, Cipso), 128.51 (d, 3JPC = 12.3 Hz, Cmeta), 132.00 (Cpara), 133.93 (d, 2JPC = 9.8 Hz, Cortho), 146.41 (1C, C4H5N2), 168.65 (d, 3JPC = 12.2 Hz, C=O), 170.39 (d, 2JPC = 13.3 Hz,P-C=C). 31P NMR (202.4 MHz, CDCl3): δ 24.47(Ph3P+-C).
References
- Maghsoodlou M. T., Habibi-Khorasani S. M., Heydari R., Hassankhani A., Marandi G., Nassiri M. and Mosaddeg E., Mol Divers, 11, 87 (2007)
- Maghsoodlou M. T., Habibi-Khorasani S. M., Niroumand U., Rostami-Charati F. and Khosrosharodi M., Phosphor, Sulfur and Silicon, 182, 647 (2007)
- Yavari I. and Karimi E., Phosphor, Sulfur and Silicon, 182, 595 (2007)
- Robiette R., Richardson J., Aggarval V. K and Harvey J. N., J. Am. Chem. Soc, 127, 13468 (2005)
- Alizadeh A. and Bijanzadeh H. R., Synthesis, 18, 3023 (2004)
- Islami M. R., Mollazehi F., Badiei A. and Sheibani H., Arkivoc, xy, 25 (2005)
- Hassani Z., Islami M. R., Sheibani H., Kalantari M. and Saidi K., Arkivoc, I, 89 (2006)
- Kalantari M., Islami M. R., Hassani Z. and Saidi K., Arkivoc, x, 55 (2006)
- Kaboudin B., Haruki T., Yamagishi T. and Yokomatsu T., Tetrahedron., 63, 8199 (2007)
- Bravo-Altamirano K., Jean-Luc. Montchamp, Tetrahedron Lett, 48, 5755 (2007)
- Benetsky E. B., Zheglov S. V., Grishina T. B., Macaev F. Z., Bet L. P., Davankov V. A. and Gavrilov K. N., Tetrahedron Lett, 48, 8326 (2007)
- Ito S., Hashino S., Morita N., Yoshifuji M., Hirose D., Takahashi M. and Kavazoe Y., Tetrahedron, 63, 10246 (2007)
- Coudray L., Abrunhosa-Thomas I. and Montchamp J. L., Tetrahedron Lett, 48, 6505 (2007)
- Morisaki Y., Ouchi Y., Naka K. and Chujo Y., Tetrahedron Lett, 48, 1451 (2007)
- Yamagishi T., Haruki T. and Yokomatsu T., Tetrahedron, 62, 9210 (2006)
- Maghsoodlou M. T., Habibi-Khorassani S. M., Rofouei M. K., Adhamdoust S. R. and Nassiri M., Arkivoc, xii, 145 (2006)
- Maghsoodlou M. T., Hazeri N., Habibi-Khorassani S. M., Saghatforoush L., Rofouei M. K. And Rezaie M., Arkivoc, xiii, 117 (2006)
- Maghsoodlou M. T., Habibi-Khorassani S. M., Heydari R. and Rostami Charati F., J.Chem. Res, 364 (2006)
- Yavari I. and Ramazani A., Phosphor, Sulfur and Silicon, 130, 73 (1997)
- Yavari I., Anary-Abbasinejad M. and Hossaini Z., Org. Biomol. Chem, 1, 560 (2003)
- Brel V. K., Synthesis, 13, 1829 (2002)
- Krawczyk Wolf W. and Sliwinski M., J. Chem. Soc. Perkin Trans 1, 1, 2794 (2002)
- Moonen K., Van. Meenen E., Verwee A. and Stevens C. V., Angewandte, 44, 7407 (2005)
- Jankowski S., Marczak J., Olczak A.and Gloka M. L., Tetrahedron Lett., 47, 3341 (2006)
- Trost M. B., Science, 254, 1471 (1991)
- Trost, M. B., Acc. Chem. Res, 35, 695 (2002)
- Hudson H. R., The Chemistry of Organophosphorus Compounds, Vol. 1. Primary, Secondary and Tertiary phosphines, Polyphosphines and heterocyclic Organophosphorus (III) Compounds, 1, pp 386-472 (Wiley, New York, 1990)
- Engel R., Synthesis of Carbon-Phosphorus Bond (CRC Press: Boca Raton, FL. 1988)
- Cadogan J. I. G., Organophosphorus Reagents in Organic Synthesis (Acade- mic Press, New York, 1979)
- Moonen K., Laureyn I. and Stevens C., Chem. Rev, 104, 6177 (2004)
- George M. V., Khetan S. K. and Gupta R. K., Adv. Heterocyclic. Chem., 19, 354 (1976)
- Burgada R., Leroux Y. and Khoshnieh Y.U., Tetrahedron Lett, 22, 3533 (1981)
- Gilchrist T. L., Heterocyclic Chemistry (Wiley, New York, 1985)







