Manuscript accepted on :April 04, 2008
Published online on: --
Plagiarism Check: Yes
Ambuj Singh1*, S. K. Prajapati, K. P. Namdeo2, V. K. Singh and S. K. Verma
1Institute of Pharmacy, Bundelkhand University, Jhansi.
2S. L. T. Institute of Pharmaceutical Sciences, G.G.U., Bilaspur, Chhatisgarh.
Abstract
Synthesis of six 2,3,6-trisubstituted Quinazolin-4-one is reported. All six compounds contain a bromine atom at position 6, phenylimino thiazolidinone group at position 2 and 3 different derivatives has been synthesized by changing X (A-1: benzaldehyde, A-2: m-chlorobenzaldehyde, A-3: o-hydroxybenzaldehyde, A-4: p-methoxybenzaldehyde, A-5: o-nitrobenzaldehyde, A-6: m-nitrobenzaldehyde). All the synthesized compounds work evaluated for their antimicrobial (against two gram positive and two gram negative microorganism) and antifungal activity (against A. niger and C. albicans).
Keywords
Antimicrobial activity; Quinazolinone derivatives
Download this article as:| Copy the following to cite this article: Singh A, Prajapati S. K, Namdeo K. P, Singh V. K , Verma S. K. Synthesis and Antimicrobial Activity of some Quinazolinone Derivatives. Biomed Pharmacol J 2008;1(1). |
| Copy the following to cite this URL: Singh A, Prajapati S. K, Namdeo K. P, Singh V. K , Verma S. K. Synthesis and Antimicrobial Activity of some Quinazolinone Derivatives. Biomed Pharmacol J 2008;1(1). Available from: http://biomedpharmajournal.org/?p=306 |
Introduction
Different types of Quinazolinones exhibit a wide spectrum of biological activities including anticancer1, antimicrobial2,5, anticonvulsant3, antitubercular4, anti-inflammatory6, anlagesic6, estrogenic7 and antiparkinsonism8 . Along 5-membered heterocycles thiazolidinone are found to be significant in their anticonvulsant and antimicrobial. Based on these findings the planned to synthesize the title compounds presuming that these chemical entities with thiazolidinone moiety in conjunction with Quinazolinone through imino bridge.
Materials and Methods
All chemicals used were AR grade , procured from various chemicals units Merck (Mumbai), Qualigens( Mumbai), Himedia (Mumbai), CDH (New Delhi). The melting points were recorded on the capillary thiel’s tube method. Purity of the compounds was checked by TLC on ready made precoated TLC plates having silica gel F28 as adsorbent using pet ether and ethyl acetate (8:2) as mobile phase. IR spectra were recorded from Perkin Elmer (KBr disc). 1H NMR spectra were obtained on the Bruker Avance 400 in D2O, CDCl3 and DMSO. Mass spectra were measured with an FAB mass spectrophotometer (Jeol SX-102).
Step-1
Synthesis of 5-bromo anthranillic acid
To a solution of (0.3M, 41.1gm) of anthranillic acid in 500ml of glacial acetic acid, 19 ml of bromine was added at a temperature of 200C. The resulting mixture was extracted with 1L of water containing 50ml of concentrated HCl, followed by filtration. 5- bromo anthranillic acid crystallized out as pale yellowish crystals by cooling the filtrate. m.p.-2120C, yield-90%, Rf – 0.11, IR (cm-1): 680 υ (C-Br str. of the ring),1698 υ (C=O str. of the ring ), 3030υ (C-H str. of the aromatic ring), 3383, 3363 υ (N-H str. of the ring), 1H NMR: 7.6-7.7 (d, 2H of –NH2), 7.2-7.3 (dd, 2H of-ArH), 6.71 (d, 1H of -ArH).
Step-2
Synthesis of 6-bromo,2-(o-aminophenyl)-3,1-benzoxazin-4(3H)-one
5-bromo anthranillic acid (.16M, 34.72gm) was dissolved in 100 ml of pyridine. To this reaction mixture o-amino benzoyl chloride (.16M, 24.8gm) was added with stirring at room temperature. Stirring continued for 30 mins at the same temperature. This reaction mixture was filtered out and collect the precipitate, which was washed with distilled water and Pet.ether 60/80 to remove the traces of pyridine. The pale creamish crystals obtained were dried at 600C. m.p.-1900C, yield- 75%, Rf – 0.65, IR (cm-1): 3068 υ (C-H str. of the aromatic ring), 1698 υ (C=O str. of the ring), 3365 3345 υ (N-H str. of the ring). 1H NMR: 7.7-7.8 (d, 2H of –NH2), 7.18-7.25 (dd, 2H of-ArH), 6.71 (d, 1H of -ArH).
Step- 3
Synthesis of 6-bromo-2-(o-aminophenyl)-3-amino-Quinazolin-4(3H)-one
6-bromo-2-(o-aminophenyl)-3-,1-benzoxazin-4(3H)-one (0.075M, 23.775gm) was refluxed with 75 mL of hydrazine hydrate for 3 hrs at 120-1300C. the reaction mixture was allowed to cool to room temperature. Pale creamish crystals developed were recrystallized from super dry ethanol. m.p.-178-1800C, yield-75%, Rf- 0.68, IR (CM-1): 3048 υ (C-H str. of the aromatic ring), 3361, 3351 υ (N-H str. of the ring), 1706 υ (C=O str. of the ring), 1316 υ (C-N str. of the ring). 1H NMR: 7.72-7.80 (dd, 2H of –ArH), 7.43-7.86 (m, 5H of –ArH), 5.65 (s, 2H of NH2), 6.18 (s, 2H of –NH2).
Step- 4
Synthesis of 6-bromo-2-(o-thiadiaminephenyl)-3-thiadiamine-quinazoline-4(3H)-one
6-bromo-2-(o-aminophenyl)-3-amino-Quinazolin-4(3H)-one (0.055M, ) was dissolved in minimum amount of dil. HCl in a round bottom flask. Ammonium thiocyanate (0.11M, 9.68gm) was then added and the mixture refluxed for 7 hrs. After cooling the product separated out as crystals which were separated and washed with cold distilled water for several times and dried. Recrystallized effected with rectified spirit. m.p.-161-165OC, yield- 90% , Rf- 0.62. IR (cm-1): 3077 υ (C-H str. of the aromatic ring), 1562 υ (C=S str. of the ring), 3363, 3353 υ (N-H str. of the ring), 1698 υ (C=O str. of the ring ), 1319 υ (C-N str. of the ring). 1H NMR: 8.35 (d, 1H of –ArH), 7.65-7.80 (dd, 2H of –ArH), 7.39-7.79 (m, 5H of –ArH), 2.21 (s, 1H of –NH), 12.45(s, 1H of –NH), 9.53 (s, 2H of NH2).
Step- 5
Synthesis of 6-bromo-2-[o-imino-(4-thiazolidinone)-phenyl]-3-imino-(4-thiazolidinone)-Quinazolin-4(3H)-one
A mixture of 6-bromo-2-(o-thiadiaminephenyl)-3-thiadiamine-Quinazolin-4(3H)-one (0.037M, 16.095gm) and fused sodium acetate (0.074M, 6.068gm) was taken in absolute alcohol (300ml) and refluxed for 10 hrs. The bulk of the solvent was reduced to about one third by distilling off the solvent under reduced pressure. Ice cold water was then added to the content. The precipitate so obtained was filtered and washed with distilled water. Rectified spirit was used for recrystallization. m.p.- 202-208OC, yield-85%, Rf = 0.69, IR (cm-1): 1506 υ (C=N str. of the ring), 679 υ (C-S-C str. of the ring). 1H NMR: 11.78 (s, 1H of –NH), 3.36 (s, 2H of -CH2), 3.75 (s, 2H of –CH2), 7.36-7.80 (m, 5H of –ArH), 8.08-8.18 (d, 2H of –ArH).
Step- 6
Synthesis of the different derivatives
A mixture of 6-bromo-2-[o-imino-(4-thiazolidinone)-phenyl]-3-imino-(4-thiazolidinone)-Quinazolin-4(3H)-one (0.005M, 2.25gm), required aromatic aldehyde (0.01M,) and anhydrous sodium acetate (0.01M) was made in glacial acetic acid and refluxed for 8 hrs. After cooling the solution was poured on ice to precipitate the product. Warm water was used to wash the precipitate. The product was recrystallized with rectified spirit.
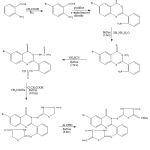 |
Scheme 1 |
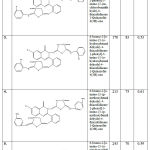 |
Table 1 : % Yield, Rf, Melting Point of compounds |
Table 2: FTIR, 1H NMR, MASS spectral data of compounds
| S. NO. | DERIVATIVES | IR (cm-1) | 1H-NMR
(δppm) |
MASS
SPECTRA |
| 1. | A-1 | ArC-H:- 3069,
N-H:- 3337, C=O:- 1697, C-S-C:- 677
|
7.68 (2H,s),
8.08-8.16 (2H, d), 7.33-7.76(15H, m) |
m/z :-737.05 |
| 2. | A-2 | ArC-H:- 3063,
N-H:- 3341, C=O:- 1703, C-S-C:-671, C-Cl:- 757,
|
7.75 (2H,s)
8.03-8.19 (2H, d) 7.32-7.4(13H, m) |
m/z :- 805 |
| 3. | A-3 | ArC-H:- 3063,
N-H:- 3341, C=O:- 1703, C-S-C:-671, OH:- 3370 |
7.70 (2H,s),9.48 (1H,s),
6.73-6.84 (2H, d), 7.25-7.60(8H, m), 8.09-8.21 (2H, d) |
m/z :-769.07 |
| 4. | A-4 | ArC-H:- 3063,
N-H:- 3341, C=O:- 1703, C-S-C:-671 OCH3:- 2919 |
7.91 (2H,s),6.94 (2H,d),
7.37-7.65(9H, m), 3.83 (3H,s), 8.03-8.16 (2H, d) |
m/z :-797.10 |
| 5. | A-5 | ArC-H:- 3063,
N-H:- 3341, C=O:- 1703, C-S-C:-671 NO2:- 1521 |
7.97 (2H, s),
6.92-6.97 (2H, d), 7.20-7.62(9H, m), 8.01-8.19 (2H, d) |
m/z :-827.05 |
| 6. | A-6 | ArC-H:- 3063,
N-H:- 3341, C=O:- 1703, C-S-C:-671 NO2:- 1529 |
7.85 (2H, s),
7.39-7.67(9H, m), 7.85 (1H, s), 8.72 (1H, s) |
m/z :-827.05 |
Table 3: Antimicrobial Activity
| S.
No. |
Compounds | Bacteria |
Fungus |
||||
| Gram-positive | Gram-negative | ||||||
| S.aureus | S.pyogen | E.coli | P.aeruginosa | A.niger | C.albicans | ||
| 1. | A-1 | + + | + + | + | + | – | + |
| 2. | A-2 | – | + | + + + | + + | – | + + |
| 3. | A-3 | + + | – | – | + + | + + + | – |
| 4. | A-4 | – | + + | + + | + + + | + + | – |
| 5. | A-5 | + + | + + | – | + | + + | + + |
| 6. | A-6 | + | + | – | + | + + | + + + |
| 7. | Standard | + + + | + + + | + + + | + + + | + + + | + + + |
| 8. | N. S. | – | – | – | – | – | – |
Antimicrobial Activity
All synthesized compounds were subjected to antimicrobial activity on agar plate using disc diffusion. The bacterial cultures used were Staphylococcus aureus, Streptococcus pyogens (gram +ive); Escherichia coli, Pseudomonas aeruginosa (gram -ive).
For antifungal activity study Aspergillus niger, Candida albicans cultures were choosen. The concentration of the compounds synthesized and the standard drug were taken as 50 µg/mL. All the drugs taken were dissolved in water and DMF.
Compound A-1 possess very good activity against S.aureus, S.pyogen and good against E.coli., P.aeruginosa.
Compound A-2 possess excellent activity against E.coli and very good against C.albicans.
Compound A-3 possess excellent activity against A.niger and very good against P.aeruginosa.
Compound A-4 possess excellent activity against P.aeruginosa and very good against S.pyogen, E.coli, A.niger.
Compound A-5 possess very good activity against S.aureus, S.pyogen, A.niger, C.albicans and good against P.aeruginosa.
Compound A-6 possess excellent activity against C.albicans, very good activity against A.niger and good against S.aureus, S.pyogen, P.aeruginosa.
Result and Discussion
Initial antimicrobial activity data for the Quinazolinone derivatives are reported in Table no.3. Along with the literature data of standard drug. Derivative A-2 showd excellent activity against E.coli, derivative A3 showed excellent potency against A.niger, derivative A4 has excellent activity against P.aeruginosa and A-6 excellent against C.albicans. All of these derivatives which mention above shows considerable inhibition against satandard drug.
Acknowledgment
The authors are thankful to Institute of Pharmacy, Bundelkhand University, Jhansi for providing the necessary facilities to carry out the research work, SAIF – CDRI, Lucknow for providing IR and Mass spectral data, SAIF – Punjab University, Chandigarh for providing 1H NMR spectral data and Institute of Microbial Technology, Chandigarh for providing the desired strains of bacteria and fungi.
References
- Raghavendra N.M., Niranjan M.S., Venkatesh P., Prashantha Kumar B.R., Gowda N.B., Sripathi M.S., Synthesis and biological activity of some substituted 2-phenyl-quinazolin-4-one, Asian Journal of Chemistry, vol.17 no.1, 57-65, 2005.
- Mishra P., Namdeo K.P., Jain S.K., Jain S., Synthesis and Antimicrobial Activity of 4- Thiazolidinones, Asian Journal of Chemisry, vol. 11 no.1, 55-58, 1999.
- Jatav V., Mishra P., Kashaw S., Stables J.P., Synthesis and CNS depressant activity of some novel 3-[5-substituted-1,3,4-thiadiazole-2-yl]-2-styryl-quinazoline-4(3H)-one, European Journal of Medicinal Chemistry, Vol. XX, 1-7, 2007.
- Bhat A.R., Shenoy G., Kotain M., Synthesis and biological activities of Mannich bases of 7-nitro-2-methyl-quinazolin-4(3H)-one, Indian Journal of Heterocyclic Chemistry, vol. 9, 319-320, 2000
- Abdel-Hamide S.G., El-Hakim A.E., Abdel-Rahman R.M., Synthesis and biological activities of some new heterocyclic compounds bearing 2-phenyl-6-iodo-quinazolinyl-4-oxy moiety part-1, Indian Journal of Heterocyclic Chemistry, vol. 5, 219-222, 1996.
- Maggio B., Daidone G., Demetrio R., Plescia S., Mantione L, Cutuli V.M., Mangano N.G., Caruso A., Synthesis and pharmacological study of ethyl 1-methyl-5-(substituted3,4-dihydro-4-oxoquinazolin-3-yl)-1H-pyrazole-4-acetates, European Journal of Medicinal Chemistry, Vol. 36, 737-742, 2001.
- Murugan V., Bhaskaran M.V., Rama Sarma G.V.S., Ramanathan M.,Suresh B., Synthesis and estrogenic screening of some new quinazolinonyl triazoles, Indian Journal of Heterocyclic Chemistry, Vol. 9, 267-270, 2000.
- Srivastava V.K., Singh S., Gulati A., Shankar K., Antiparkinsonian agents from Quinazolinylthiazolidinones and Azetidinones, Indian Journal of Chemistry, Vol. 26B, 652-656, 1987.
- Mishra P., Gajbhiye A., Jain S.K., Synthesis and antibacterial activity of some 4- thiazolidinone (Part-1). Oriental Journal of Chemistry, Vol. 12 No.(3), 325-326, 1996.
- Astik R.R., Acharya J.N., Joshi G.B., Thakar K.A., Studies on Thiazolidinones: Part II, Journal of Indian Chemistry, Vol. LIII, 272-273, 1976.
- Tiwari N., Chaturvedi V., Nizamuddin, Synthesis and fungicidal activities of some 2-aryloxymethyl-1,3,4-thiadiazolo[2,3,-b]-quinazolin-4-one and 2-aryloxymethyl-5-substituted-1,3,4-thiadiazolo[3,2,-a]-1,3,5-triazine-7-thiones, Indian Journal of Chemistry, Vol. 28B, 200-202, 1989.
- Bennur S.C., Mahadev B.T., Laddi U.V., Somannavar Y.S., Hariholimath V., Badiger V.V., Synthesis and biological activity of 2-(N,N-dialkyl amino/-piperidino/morpholino)-methyl-7-halothiazolo (2,3-b)-quinalzolin-5-ones, Indian Journal of Heterocyclic Chemistry, Vol. 7, 39-42, 1997.







