S. K. Verma1, R. Irrchhaiya2 , K. P. Namdeo2, V. K. Singh2, A. Singh2 and S. Khurana3
1Institute of Pharmacy, Bundelkhand University, Jhansi.
2S.L.T Institute of Pharmaceutical Sciences G.G.U., Bilaspur.
3I.T.S. Paramedical college, Ghaziabad.
Abstract
Some new 6-phenyl–Pyridazine-3-One were synthesized by hydrazinolysis of appropriate b-(substituted aryl) propionic acid, there were converted into corresponding Bis-pyridazinone (IIIa toIIId). All these synthesized compounds were characterized on the bases of spectral data and evaluated for their antimicrobial activity.
Keywords
Pyridazine-3-One; Antibacterial activity; Antifungal activity
Download this article as:| Copy the following to cite this article: Verma S. K, Irrchhaiya R , Namdeo K. P , Singh V. K , Singh A , Khurana S. Synthesis and Antimicrobial Activity of some Pyridazinone Derivatives. Biomed Pharmacol J 2008;1(1). |
| Copy the following to cite this URL: Verma S. K, Irrchhaiya R , Namdeo K. P , Singh V. K , Singh A , Khurana S. Synthesis and Antimicrobial Activity of some Pyridazinone Derivatives. Biomed Pharmacol J 2008;1(1). Available from: http://biomedpharmajournal.org/?p=334 |
Introduction
Pyridazinones have been reported to possess variety of biological activities like antinociceptive1, anticancer2, and anticonvulsant3, antithrombotic4, and antiinflammatory5-6 activity. In present work we report the synthesis of compounds and their antimicrobial activity which is not done earlier and is quite significant.
Reaction between benzene with succinic anhydride in the presence of aluminium chloride produced benzoyl propionic acid (step-I). The product obtained from step-I react with hydrazine hydrate to produced 6-phenyl-2,3,4,5-tetrahydro Pyridazin-3-one (step-II). After this the product obtained from step-II react with different aromatic benzaldehydes in the presence of Acetic anhydride to produce different derivatives (IIIa to IIId).
Materials and Methods
Melting points were determined by open capillary tube method in liquid paraffin bath. All the reactions were monitored by thin layer chromatography (TLC) using Toluene: Ethyl acetate: Formic acid (5:4:1) as solvent system. IR spectra (Kbr disc) were recorded on FTIR Perkin Elmer. 1H NMR spectra were recorded in DMSO using Bruker Avance-II 400 MHz NMR spectrophotometer. The chemical shifts were expressed in terms of ppm downfield from TMS. And the mass spectra were recorded on Jeol Sx-102 (FAB) Mass Spectrometer.
Synthetic Study
Step-I
Synthesis of benzoyl propionic acid
A mixture of benzene (30ml) and anhydrous aluminum chloride (0.10M) was refluxed on a water bath under anhydrous condition followed by addition of succinic anhydride (0.10M) in small quantities with continues stirring. Stirring and heating were continued for 4 hrs and the contents after leaving overnight at room temp were poured into ice cold hydrochloric acid (2.5% v/v) followed by steam distillation. The aqueous Solution was concentrated to a small volume by evaporating on a water bath to obtain the crude compound-(I). It was purified by dissolving in 5% w/v sodium bicarbonate solution, followed by extraction with ether. The aqueous layer on acidification with dil. hydrochloric acid gave benzoyl propionic acid, crystallized from aq. ethanol.
M.P.1220C, Yield 70% , Rf 0.65
IR (cm-1): 3250 (OH), 1720 (C=O), 1H-NMR (δppm): 3.32(2H,m), 7.47(2H,m), 7.63(2H,m), 7.97(2H,m)
Step-II
Synthesis of 6-phenyl-2,3,4,5-tetrahydro pyridazin-3-one
Compound-I (0.1M) was refluxed for 8hrs with hydrazine hydrate (1ml) in ethanol (25ml). The reaction mixture was concentrated and then poured into ice cold water to obtain compound-(II) which was crystallized from ethanol.
M.P. 2480C, Yield 72% , Rf 0.60
IR (cm-1): 3350 (NH), 1685 (C=O), 1H-NMR (δppm): 5.7(1H,d), 6.49(1H,d), 7.0(4H,s), 7.5(3H,m), 7.9(2H,m).
Step-III
Compound-II (0.02M) was reflux with different aromatic benzaldehydes (0.01M) in the presence of acetic anhydride (10ml) for 26hrs and then poured into ice cold water to get compound-(IIIa-IIId) which was also crystallized from ethanol. Similarly other derivatives also prepared by the above method.
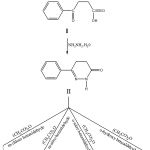 |
Scheme 1 Click here to View scheme |
Antimicrobial activity
All the synthesized compound were screened for their antibacterial activity at concentration 50µg/ml against Escherichia coli, Pseudomonas aureoginosa (gram-ve) using Gentamycin as standard drug where as Ceftizoxime was used as standard against Staphylococcus aureus where as ampicilline was used as standard against Staphylococcus pyogen (gram +ve) by disc diffusion method. The zone of inhibition was measured and compared againt standard.
Compound IIIa shows excellent activity against S.pyogen and E.coli. Compound IIIb shows good activity against S.pyogen and S.aureus and it doesn’t show any activity against P.aureginosa and E.coli. Compound IIIc shows good activity against S.pyogen and P.aureginosa is not active against S.aureus and E.coli. Compound IIId shows very good activity against S.aureus and good activity against P.aureginosa while no activity against S.pyogen and E.coli.
The same compound were also tested for antifungal activity against Aspergillus niger and Candida albicans using Fluconazole as standard drug.
Compound IIIa shows good activity against A.niger and C.albicans where as IIIb and IIIc don’t show any activity against A.niger and C.albicans. Compound IIId shows very good activity against A.niger and C.albicans.
Results and Discussion
The derivatives were synthezied as shown in reaction scheme friedel-crafts acylation of appropriate hydrocarbons with succinic anhydride in the presence of aluminium chloride to get β-(substituted aryl ) propionic acid (I) cyclization of β-(substituted aryl ) propionic acid with hydrazine hydrate to from pyridazinones-(II). On treatment of compound-(II) with acetic anhydride and corresponding aromatic benzaldehydes. The desired derivatives (viz: IIIa, IIIb, IIIc, IIId) where produced as shown and their structure were shown in Table-1. From the antimicrobial screening data it has been found that derivative IIIa have excellent antibacterial activity against both Gram +ve ( S.pyogen ) and Gram –ve (E.coli) strain where as derivative IIId has very good antifungal activity.
Table 1: Antimicrobial Activity
| S.
No. |
Compounds | Bacteria |
Fungus |
||||
| Gram-positive | Gram-negative | ||||||
| S.Pyogen | S.Aureus | P.Aeruginosa | E.Coli | A.Niger | C.Albicans | ||
| 1. | IIIa | + + + | + + | + + | +++ | + | + |
| 2. | IIIb | + | + | – | – | – | – |
| 3. | IIIc | + | – | + | – | – | – |
| 4. | IIId | – | + + | + | – | + + | ++ |
| 5. | Standard | + + + | + + + | + + + | + + + | + + + | + + + |
| 6. | Control | – | – | – | – | – | – |
Excellent : +++ , Very Good : ++ , Good : + , No Activity : – , Control : N.Saline(0.9%w/v)
Ampicillin was taken as std drug against S.Pyogen while Ceftizoxime was taken as std drug against S.Aureus. While as Gentamycin was taken as std drug against P.Aeruginosa & E.Coli. While as Fluconazole was taken as std drug against A.Niger & C.Albicans
 |
Table 2: Structure and Physiochemical data of derivatives |
Table 3 : Characterization of derivatives
| S.No. | Derivatives | IR (cm-1) | 1H-NMR (δ ppm) | Mass Spectral data (m/z) |
| 1 | IIIa | C = O : 1683, C-H: | 7.52 (6H,m), | 466.12 |
| 2923, C=N : 1623, C – | 7.98 (4H,m), | |||
| Cl : 712, 1313 | 6.98 (1H, s), | |||
| 5.7 (2H,s), 6.49 | ||||
| (2H,s) | ||||
| 7.27 – 7.52 (4H,m) | ||||
| 2 | IIIb | Ar- C – H : 3100, | 7.52 (6H,m), | 477.14 |
| 3084, | 7.98 (4H,m), | |||
| C = O : 1684, | 6.98 (1H,s), | |||
| Ar – NO2 : 1347, 1523, | 5.7 (2H,s), | |||
| C – H : 2923 | 6.49 (2H,s), 7.59- | |||
| 8.49 (4H,m) | ||||
| 3 | IIIc | Ar- C – H : 3100, | 7.52 (7H,m), | 478.10 |
| 3084, | 7.98(4H,m), 6.98 | |||
| C = O: 1684, | (1H,s), 5.7 (2H,s), | |||
| Ar- NO2 : 1347, 1523 | 6.49 (2H,s), 6.71 – | |||
| C – H : 2923, | 7.96 (3H,m) | |||
| 4 | IIId | Ar- C-H : 3100, 3084, | 7.52 (6H,m), 7.98 | 448.15 |
| C = O : 1684, | (4H,m), 6.98 (1H,s), | |||
| C – H : 2923, | 5.7 (2H,s), 6.49 | |||
| O – H : 3373, | (2H,s), 9.68 (1H,s) | |||
| C – O : 1224 | 6.83 – 7.09 (4H, m) |
Acknowledgements
The authors are thankful to Institute of Pharmacy, Bundelkhand university, jhansi for providing facilities to conduct out the experiments, SAIF, CDRI lucknow for providing IR and mass data, SAIF, P.U. chandigarh for providing 1H NMR data and institute of microbial technology, chandigarh for providing desired strains of bacteria and fungi.
References
- Pieretti.S, Pia V.D, Matucci.R, Giovannoni.M. Synthesis of 3-(2H)- Pyridazinone derivative and their antinociceptive activity. J. Of life sciences. 65, 1381-1394 (1999),
- Xup,Wang S.Y, Liu W.Q. Synthesis and anticancer activity of 6-(substituted phenyl) – 3 (2H) pyridazinones. J.Of Agric food Chem. 50, 357-3760 (2002).
- Rubat C, Coudert P, Refouvelet B, Tronche P, Bastide P. Synthesis and anticonvulsant activity of 3-oxo-5-substituted Benzylidene-6-methyl-(4H)-2-Pyridazinylacetamides and 2-Pyridazinylacetylhydrazides. Chem. Pharm. Bull. 38(11), 3009-3013 (1990). Mikashima H, Nakao T, Kazuhiro G. Synthesis of new Pyridazinone derivatives and their antithrombotic activity. J. Of Thrombosis Research. 35, 589-609 (1984).
- Siddiqui A.A, Kushnoor A, Wani S.M. Synthesis and antiinflammatory activity of 6-(Substituted aryl)-2,3,4,5-tetrahydro-3-thiopyridazinones. Indian J. Of Hetrocyclic Chem. 13, 257-260 (2004).
- Khan M.S.Y, Siddiqui A.A. Synthesis and antiinflammatory activity of some 6-aryl-2,3,4,5-tetrahydro-3-Pyridazinones. Indian J.Of Hetrocyclic Chem. 39B, 614-619 (2000).
- Mogilaiah K, Kankaiah G. Synthesis of 6-aryl-2-(2-phenyl-1,8-naphthyridine-3-carbonyl)-4,5-dihydro-pyridazin-3(2H)-ones. Indian J. Chem. 42B, 658-660 (2003).
- Sumakanth M, Malla R.V, Krishnan V.S.H, Sastry B.S. Synthesis and pharmacological evaluation of new 2,6-disubstituted-2H-Pyridazin-3-ones. J.Of Pharmaceutical Research. 6, 166-169 (2007).
- Siddiqui A.A, Khusnoor A, Ahmad S.R. synthesis and pharmacological activity of 4-substituted benzoyl methyl Pyrazolidin-3-ones. Indian drug. 2, 127-132 (2005).
- Siddiqui A.A, Khusnoor A, Ahmad S.R. synthesis and pharmacological activity of 4-substituted benzoyl methyl Pyrazolidin-3-ones. Indian drug. 2, 127-132 (2005).







