Ravindra Kumar, Birbal Bajia and Y. K. Srivastava*
P. G. Department of Chemistry, M.P. Government College Chittorgarh.
Abstract
Pyrazoline derivatives have their importance due to a variety of industrial applications and wide range of biological activities change in substitution pattern at 1-N position of pyrazoline ring may alter the biological activities of pyrazolines. Therefore some new 1-N substituted pyrazoline derivatives were synthesized using microwave assisted method. The condensation of chalcone with hydrazine gave 3, 5-diaryl-2 pyrazolines which were treated with chloro acetyl chloride followed by reaction with diethyl amine to afford corresponding 1-N substituted 3, 5-diaryl pyrazolines in 80-85% yield. The synthesized compounds were screened for their anti bacterial activities against gram negative and gram positive bacteria.
Keywords
Pyrazoline; microwave assisted; anti bacterial activity
Download this article as:| Copy the following to cite this article: Kumar R , Bajia B , Srivastava Y. K . Synthesis and Antibacterial Activity of some Pyrazolines using more Technique. Biomed Pharmacol J 2008;1(1). |
| Copy the following to cite this URL: Kumar R , Bajia B , Srivastava Y. K . Synthesis and Antibacterial Activity of some Pyrazolines using more Technique. Biomed Pharmacol J 2008;1(1). Available from: http://biomedpharmajournal.org/?p=294 |
Introduction
In the last few years there has been a growing interest in the use of microwave irradiation in the chemical reactions. Spectacular results have been obtained giving clear indication on the potentialities and advantage of this technique compared to conventional heating method1-3. Virtually all types of thermally driven reactions can be accelerated by microwave. The use of such no conventional reaction conditions reveal several advantages like a shorter reaction time, increased yield, cleaner reaction, selectivity in the reaction and easy workup. Thus microwave assisted synthesis becomes a part of green chemistry4-5. The use of domestic microwave oven in this regard is now a well established procedure in MORE Chemistry5.
Pyrazoline derivatives have been studied extensively because of their ready accessibility diverse chemical reactivity and variety of industrial applications. Variously substituted 2-pyrazolines can be effectively utilized as antimicrobial, anticonvulsant, cardiovascular, anti-inflammatory and antidepressant agent6-10. In additional pyrazolines have played a crucial role in organic synthesis11-12.
In view of interesting properties associated with 2-pyrozoline in the present investigation we report synthesis of some N-substituted 2-pyrozolines using microwave irradiation 16-17
Material and methods
All the melting points reported are uncorrected and were taken in open capillaries. The progress of reaction and purity of products was checked by TLC using silica gel–G as adsorbent and benzene-ethyl acetate (9:1) as eluent. The IR spectra were recorded on Perkins Elmer spectrometer using KBr pellet. The 1H NMR spectra were taken on brucker –DRx 300 MHz spectrometer using CDCl3. as solvent and TMS as internal standard (chemical shift in δ ppm). Mass spectra were recorded on Jeol-SX-102 mass spectrometer using m-nitro benzyl alcohol as matrix. The matrix peaks appear at m/z 107,136,154 and 289.
Experimental
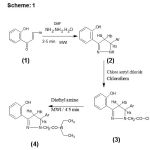
Synthesis of 3, 5-diaryl-2-pyrazolines (2a-g)
To slurry of o-hydroxychalcone (0.01mole) in DMF (10ml), hydrazine hydrate (0.015mole) was added. The reaction mixture was irradiated in microwave oven for 2-3 minutes. It was then cooled to room temperature. The residue obtained was washed with water and crystallized from ethanol as colorless crystals (2a-g).
Synthesis of 1- chloro acetyl 3, 5-diaryl-2-pyrazoline (3a-g)
3, 5-diaryl-2-pyrazoline (0.01mole) in 15ml of chloroform and added chloro acetyl chloride (0.01mole) drop wise and irradiated under microwave for 30 seconds at180 watt. power. The excess solvent was evaporated under reduced pressure the solid washed with water and crystallized in ethanol as colorless crystals (3a-g.)
Synthesis of 1–(N, N-diethyl amino acetyl)-3-(2-hydroxyphenyl)-5 aryl pyrazolines (4a-g)
A mixture of compound 3 (0.01mole) and diethyl amine (0.02mole) was subjected to microwave irradiation for 4-5 minutes with occasional disruption of 30 seconds. After completion of reaction as indicated by TLC the residue obtained was washed thoroughly with water, dried and crystallized from ethanol as colorless crystals (Table-1).
Table 1: Physical data of synthesized compounds (3a-g)
| Compd. | Ar | Molecular formula | m. p.
(0C) |
Yield (%) |
Reaction time (min.) |
| 3a | Phenyl | C25H29O4N3 | 169 | 80 | 3.5 |
| 3b | 4-Methoxyphenyl | C26H31O5N3 | 192 | 70 | 4.0 |
| 3c | 3,4-Dimethoxyphenyl | C27H33O6N3 | 178 | 75 | 4.5 |
| 3d | 3,4,5-Trimethoxyphenyl | C28H35O7N3 | 186 | 85 | 4.0 |
| 3e | 4-Chlorophenyl | C25H28O4N3 Cl | 188 | 80 | 3.5 |
| 3f | 4-Dimethylaminophenyl | C27H34O4N3 | 196 | 60 | 4.0 |
| 3g | 4-Hydroxyphenyl | C25H29O5N3 | 180 | 72 | 4.5 |
Antibacterial Activity
All the synthesized compounds ware screened for their antibacterial activity against gram positive S. aureus and S. albus and gram negative E.Coli K… pneumoneae and P. vulgaris organism. In the determination of antibacterial activity peptone nutrient broath was used, the media was used for gram positive organism by adding 2% mecconkey agar to the nutrient broath and for gram positive organism it was prepared by adding 10% sheep blood to 2% nutrient agar.
 |
Scheme 1 |
Paper disc diffusion method was followed by using special microbial filter paper disc. The compounds were screened at maximum concentration of 250μg/ml in DMF. Results were compared with standard drug Amicacin at 30μg/disc. The zone of inhibition produced by each compounds was measured in mm. (Table-2)
Table 2: Antibacterial activity of synthesized compounds (3a-g)
| S.No. | Compound | Zone of inhibition in mm. | ||||
| S. aureus | S. albus | E. coli | K. pneumoniae | P. vulgaris | ||
| 1 | 3a | 10 | 18 | 10 | 14 | 14 |
| 2 | 3b | 09 | 19 | 08 | 14 | 13 |
| 3 | 3c | 12 | 16 | 10 | 14 | 14 |
| 4 | 3d | 10 | 14 | 13 | 18 | 13 |
| 5 | 3e | 11 | 15 | 10 | 14 | 10 |
| 6 | 3f | 13 | 12 | 09 | 09 | 12 |
| 7 | 3g | 10 | 18 | 10 | 18 | 14 |
| Amicacin | 22 | 24 | 14 | 20 | 22 | |
Results and discussion
During our study under microwave induced reaction condition the product obtained was identified as 1–(N, N-diethyl amino acetyl)-3-(2-hydroxyphenyl)-5 aryl pyrazolines. The structure of the compound was confirmed on the basis of elemental analysis and spectral data. The IR spectra of compounds showed absorption band at 3430 cm-1 (broad peak -OH), 2965 cm-1 (C-H str. of aromatic ring), 2837 cm-1 (C-H str. Of –CH2– group), 1700-1680 cm-1 (C=O str.), 1230-1220 cm-1 (C=N str.) and 1130-1120 cm-1 (N-N str.). The 1 H NMR spectra showed signal as double doublet at δ 2.24-2.30 (C-Ha), 2.54-2.60 (C-Hb), 4.86-4.90 (C-Hx) confirming the presence of ABX pattern of pyrazoline ring. Aromatic protons gave multiplate at δ 6.51-7.10 and OH proton gave a singlet at δ 10.09. The methylene proton gave singlet at 1.70 and ethyl proton shows a quatrate at 3.2-3.4 and a triplet at1.2-1.3. The mass spectra of synthesized compounds gave molecular ion peak corresponding to their molecular mass besides other peaks.
The antibacterial screening results show that all compounds have excellent activity against S. albus, K. pneumonia and E coli and moderate active active against S. aureus and P. vulgaris.
Acknowledgment
Authors are thankful to Director SAIF CDRI, Lucknow for spectral analysis.
References
- Caddick S., Tetrahedron, 51, 10403 (1995).
- Verma R.S., Green Chemistry, 43, (1999).
- Galena,A. Chem. Soc. Rev., 26, 233 (1997).
- Langa F., Delacruz P. and Delahazu A., Contemporary Org. Synth., 373 (1997)
- Bose A.K., Manhas M. S., Ghosh M. and Shah M.. J, Org. Chem., 56, 6968 (1991).
- D Azarifar. and Shaebanzadeh M.,Molecules, 7, 885 (2002).
- Archana V.K., Shrivastava R.C. and Kumar A., Indian J. Chem.41B, 1310 (2002).
- Malhotra V., Pathak S., Nath R., Mukharjee D. and Shankar K., Indian J.Chem. 41B, 1310 (2002).
- Bansal E.,J Ram., Shrivastav V.K. and Kumar A., Indian J. Pharma Sic., 61B, 385, (1999).
- Palaska,E. M Aytemir., Uzbay T. and Eros D., Eur.J.Med.Chem. 36, 639 (2001).
- Illinova E.I., Marcos M., Klimova T.B., Cecilio A.T., Ruben A.T. and LenaR., J.Organometallic Chem., 106, 585 (1999).
- Padamavathi V., Sumathi R.P., Chandrashekher B.N. and Bhaskerreddy D., J.Chem. Res., 610 (1999).
- Barry A.L. in “Antimicrobial susceptibilityTest” Principle and practice, Lea & Febiger, Philadelphia, PA, USA, 180 (1976).
- Seely H.W. & Vandemark P.J., “Microbs in action A laboratory manual of Microbiology,” D.B.Taraporewala, Sons & Co. Bombay PP 55-80 (1975).
- Subbaraju G.V., Nayakulu A.R. and Parmeswara D., Indian J.Heterocyclic chem., 4, 87 (1994).
- Gurupadayya B.M., Gopal M., Basavaraj Padmashali and Vaidya V.P., J.Heterocyclic Chem.15, 169-172(2005)
- Tyagi Mirdula, Srivastava V. K., Chandra Ramesh, Kumar Ashok, Indian J. chem.., 41B,2367-2370 (2002)







