Abhishek Bansal¹*,Sanjay Singh¹ , M. A. Saleem² and Sarim Imam³
¹Sidhartha institute of Pharmacy, Dehradun.
²Luqman College of Pharmacy, Gulbarga .
³Azad Institute of Pharmacy and Research, Lucknow.
Abstract
Microemulsion based emulgel formulations of valdecoxib was developed by using ethyl oleate (EO) as oil, tween-80 in different proportions with propylene glycol (PG) as surfactant/ co-surfactant along with water and incorporated into carbopol 940 as gellant.Microemulsions were characterized for optical birefringence, globule size, polydispersity andconductivity. All microemulsion were stable, transparent, oil in water type and in globulesize range of 12.4-15.3nm. The prepared emulgels were evaluated for pH, drug content,viscosity, in vitro drug release study using dialysis membrane, and skin irritationtest. The pH and drug content of emulgels was in acceptable range of topical application. The viscosity was increased as the proportion of tween-80 and ethyl oleate increased. The in vitro release of valdecoxib was also increased as the proportion of tween-80 increased. Therelease was in the order of 3:1>2:1>1:1 ratios of tween-80 to PG. The release obeys Higuchi’s diffusion equation and follows first order kinetics.All the emulgels were free from skin irritation and were stable at room temperature. From the study it can be concluded that microemulsion based emulgel formulation containing tween-80: PG in the proportion of 3:1 was the best formulation.
Keywords
valdecoxib; microemulsion; emulgel; in vitro release; partition determination
Download this article as:| Copy the following to cite this article: Bansal A , Singh S , Saleem M . A , Imam S .Preparation and Evaluation of Valdecoxib Emulgel Formulations. Biomed Pharmacol J 2008;1(1). |
| Copy the following to cite this URL: Bansal A , Singh S , Saleem M . A , Imam S .Preparation and Evaluation of Valdecoxib Emulgel Formulations. Biomed Pharmacol J 2008;1(1). Available from: http://biomedpharmajournal.org/?p=264 |
Introduction
Microemulsion typically consist of oil, surfactant, cosurfactant and aqueous phase,which is transparent, thermodynamically stable and has a droplet size < 0.15 nm and does not have the tendency to coalesce (Kreilgaard, 2002). Microemulsion has several advantages such as enhanced drug solubility, good thermodynamic stability, ease of manufacturing and enhancement effect on transdermal delivery over conventional formulation (Lawrence and Rees, 2000; Gasco, 1997). Microemulsion-based topical gels of either oil-in-water (o/w) or water-in-oil type produce a transparent product that has micronsized oil droplets that do not coalesce. Delivery of drugs using these microemulsion-based gels through skin increases the local/systemic delivery of the drug by different mechanisms that make them suitable vehicles for the delivery of NSAIDs1. An additional advantage of these microemulsion-based gels is that drugs that with poor solubility in water and ethanol can be incorporated into these systems in sufficient quantity to enhance drug delivery. Valdecoxib was used as a highly lipophilic model drug in the present study. Valdecoxib is a nonsteroidal anti-inflammatory drug that acts by inhibition of cycloxygenase II (COX-II) and is used in the treatment of inflammation and arthritis. The adverse effects are GI disturbances and hypertension after oral use. In the present study, an attempt has been made to develop emulgel formulations of valdecoxib so as to reduce adverse effects and to enhance percutaneous absorption.
Materials
Valdecoxib was kind gift sample from Arti Drugs Ltd Mumbai, and Unichem Lab Ltd Ghaziabad. Ethyl oleate was purchased from Loba Chemie, Mumbai. Carbopol 940 LR, Tween-80, Propylene Glycol, Triethanolamine LR were purchased from SD Fine Chem Ltd. Mumbai. All other chemicals were of analytical grade.
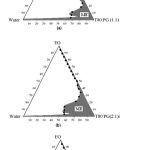 |
Figure 1: Pseudoternery phase diagrams of the oil-surfactant/ cosurfactant-water system at (a) 1:1 (b) 2:1 and (c) 3:1 weight ratios of tween-80: PG at 25ºC (ME represents microemulsion region) |
Table 1: Solubility of valdecoxib in various oils and oily mixtures
(mean±SD, n=3)
| Sl. No. |
Vehicle |
Solubility (mg/ml) |
| 1. | Ethyl oleate | 0.173±0.10 |
| 2. | Oleic acid | 2.234±0.20 |
| 3. | IPM | 0.046±0.10 |
| 4. | Propylene glycol | 0.758±0.13 |
| 5. | Oily mixture containing ethyl oleate | 2.422±0.14 |
| 6. | Oily mixture containing oleic acid | 4.662±0.23 |
| 7. | Oily mixture containing IPM | 0.160±0.19 |
Methods
Screening of oils for microemulsions2
To find out the suitable oil which can be used as the oil phase in microemulsion and provide excellent skin permeation rate of valdecoxib, the solubility of valdecoxib in various oils including IPM, OA and EO was measured. The solubility of valdecoxib in oily mixtures containing 1:10:5 ratio of oil: tween-80: PG was also measured. An excess amount of valdecoxib was added to each oil and oily mixtures and then mixed by stirring for 72 hours at 25ºC. The equilibrated sample was centrifuge to remove the excess amount of valdecoxib undissolved and filtered through a membrane filter. Finally diluted with methanol and the concentration of valdecoxib was determined by UV-visible spectrophotometer at 235 nm.
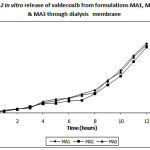 |
Figure 2: In vitro release of valdecoxib from formulations MA1, MA2 and MA3 through dialysis membrane |
Table 2: Characterization parameters of valdecoxib microemulsions
| Sl. No. | Formulation code | Conductivity (ms/cm) (mean±SD, n=3) | Droplet Size (nm) (mean±SD, n=3) | Polydispersity Index |
| 1. | MA1 | 83.26±0.83 | 14.4±0.30 | 0.123 |
| 2. | MA2 | 87.70±1.81 | 15.2±0.60 | 0.120 |
| 3. | MA3 | 82.80±3.12 | 15.3±0.30 | 0.123 |
| 4. | MB1 | 200.70±2.94 | 13.6±0.50 | 0.152 |
| 5. | MB2 | 249.47±0.30 | 13.8±0.20 | 0.151 |
| 6. | MB3 | 178.97±1.90 | 13.5±0.60 | 0.147 |
| 7. | MB4 | 142.30±1.73 | 14.2±0.30 | 0.147 |
| 8. | MC1 | 91.36±0.28 | 12.4±0.20 | 0.162 |
| 9. | MC2 | 113.30±0.45 | 12.6±0.50 | 0.154 |
| 10. | MA1C | 83.93±0.73 | 14.7±0.60 | 0.153 |
| 11. | MB1C | 203.17±0.15 | 13.4±0.80 | 0.172 |
| 12. | MC1C | 95.33±0.32 | 12.6±0.60 | 0.161 |
| 13. | MA1M | 82.10±1.41 | 14.6±0.50 | 0.858 |
| 14. | MB1M | 198.70±1.61 | 13.3±0.30 | 0.777 |
| 15. | MC1M | 98.40±0.23 | 12.4±0.10 | 0.774 |
Construction of pseudo-ternary phase diagram3
In order to find out the concentration range of components for the existence range of microemulsions, pseudo-ternary phase diagrams were constructed using water titration method at ambient temperature (25ºC). These phase diagrams were prepared with the 1:1, 2:1 and 3:1 weight ratios of tween-80 to PG respectively. For each phase diagram at specific surfactant / co-surfactant weight ratio, the ratios of EO to the mixture of surfactant and cosurfactant were varied as 0.5:9.5, 1:9, 1.5:8.5, 2:8, 2.5:7.5, 3:7, 3.5:6.5, 4:6, 4.5:5.5, 5:5, 5.5:4.5, 6:4, 6.5:3.5, 7:3, 7.5:2.5, 8:2, 8.5:1.5, 9:1, 9.5:0.5. The mixtures of oil, surfactant and co-surfactant at certain weight ratios were diluted with water drop-wise, under moderate magnetic stirring. After being equilibrated, the mixtures were assessed visually anddetermined as being microemulsions, crude emulsions or gels.
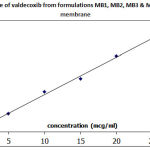 |
Figure 3: In vitro release of valdecoxib from formulations MB1, MB2, MB3 and MB4 through dialysis membrane |
Table 3: Formulation of valdecoxib emulgels
| Ingredient (% w/ w) | Formulation Code | ||||||||
| MA1 | MA2 | MA3 | MB1 | MB2 | MB3 | MB4 | MC1 | MC2 | |
| Valdecoxib | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Ethyl oleate | 3.00 | 7.00 | 11.00 | 3.00 | 6.00 | 9.00 | 14.00 | 4.00 | 7.00 |
| Tween-80 | 33.00 | 32.00 | 32.00 | 37.20 | 36.00 | 35.21 | 38.00 | 54.00 | 49.50 |
| Propylene glycol | 33.00 | 32.00 | 32.00 | 18.60 | 18.00 | 17.60 | 19.00 | 18.00 | 16.50 |
| Water | 31.00 | 26.00 | 24.00 | 41.00 | 40.00 | 38.00 | 29.00 | 24.00 | 26.00 |
| Carbopol 940 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 |
Characterization of microemulsion4
Optical birefringence
The microemulsion was checked both visually and using crosspolarizers for optical isotropy to confirm absence of other phases.
Determination of globule size and polydispersity index
All measurements were performed in triplicate on a Beckman N4 plus submicron particle size analyzer at a temperature of 25±2ºC and at 90º to the incident beam applying the principle of photon correlation spectroscopy on sample.
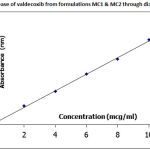 |
Figure 4: In vitro release of valdecoxib from formulations MC1 and MC2 through dialysis membrane |
Table 4: Evaluation parameters of valdecoxib emulgels
(mean±SD, n=3)
| Sl. No. | Formulation code | pH | Drug content (%) |
| 1. | MA1 | 5.1±0.15 | 97.48±0.54 |
| 2. | MA2 | 5.2±0.15 | 96.63±0.35 |
| 3. | MA3 | 5.2±0.05 | 96.18±1.70 |
| 4. | MB1 | 5.2±0.20 | 98.42±0.20 |
| 5. | MB2 | 5.1±0.08 | 97.16±0.40 |
| 6. | MB3 | 5.3±0.11 | 97.12±0.38 |
| 7. | MB4 | 5.4±0.20 | 98.46±0.07 |
| 8. | MC1 | 5.1±0.05 | 98.83±0.15 |
| 9. | MC2 | 5.5±0.10 | 97.43±0.23 |
| 10. | MA1C | 5.1±0.05 | 97.89±0.34 |
| 11. | MB1C | 5.2±0.20 | 98.78±0.15 |
| 12. | MC1C | 5.0±0.11 | 98.33±0.07 |
| 13. | MA1M | 5.1±0.10 | 98.2±0.08 |
| 14. | MB1M | 5.3±0.05 | 98.74±0.42 |
| 15. | MC1M | 5.2±0.06 | 98.65±0.40 |
Conductivity measurement
To characterize the type (o/w or w/o) of the microemulsions, the conductivity of the microemulsions was checked by autoranging conductivity meter (Equip-Tronics EQ 667).
Development of valdecoxib emulgels5
Valdecoxib 1%w/w was added to the selected clear microemulsions obtained from pseudoternery phase diagrams. Weighed quantity of carbopol was soaked in the microemulsion system by stirring to disperse the carbopol in the microemulsion and finally neutralize with sufficient triethanolamine amine to obtained emulgels.
Evaluation of emulgels
Determination of pH, drug content and viscosity6
2.5 gm of the gel formulation was dispersed in 25 ml of distilled water and the pH was determined by using digital pen pHmeter. For determination of drug content, about one gram of the gel was weighed in a 100mL volumetric flask and dissolved in methanol; it was diluted appropriately and analyzed on a Shimadzu UV-Visible spectrophotometer at 235nm . The viscosity was determined by using Brookfield rheometer (LVDV-III+) at an optimum speed of 2 rpm with spindle No. CP 52.
Table 5: Rheological parameters of valdecoxib emulgels
(mean±SD, n=3)
| Sl. No. | Formulation code | Viscosity (CPS) | Spreadability (gm-cm/sec) | Extrudability |
| 1. | MA1 | 527.36±26.41 | 11.30±0.38 | ++ |
| 2. | MA2 | 854.86±5.03 | 6.73±0.40 | ++ |
| 3. | MA3 | 950.44±8.76 | 5.86±0.10 | + |
| 4. | MB1 | 475.60±4.43 | 11.86±0.36 | ++ |
| 5. | MB2 | 642.21±10.15 | 8.57±0.06 | ++ |
| 6. | MB3 | 789.21±11.84 | 6.88±0.00 | ++ |
| 7. | MB4 | 951.58±31.95 | 5.44±0.04 | + |
| 8. | MC1 | 475.76±3.82 | 11.52±0.16 | ++ |
| 9. | MC2 | 988.45±16.03 | 5.21±0.22 | + |
++ Good, + Satisfactory
In Vitro diffusion study7
In vitro release study of valdecoxib from emulgel was done by using modified beaker method. By using cellophane membrane: 1 gm of emulgel formulation equivalent to 10 mg of valdecoxib was spread uniformly on the surface of cellophane membrane (previously soaked in water for overnight). The diffusion medium used was 100 ml distilled water containing 0.05% SLS contained in100 ml beaker. The cellophane membrane acts as a barrier between the gel phase and distilled water containing 0.05% SLS (sink phase). The contents were stirred using magnetic stirrer at 100±10 rpm. A quantity of 1 ml sample was withdrawn from receptor fluid at the time interval of 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 hours. The released drug was estimated by using Shimadzu UV-visible spectrophotometer at 256 nm.
Discussion
To develop microemulsion formulations, the optimum oil needs to be chosen. The solubility of valdecoxib in various oils and in oily mixtures is shown in Table 1. The solubility of valdecoxib was highest in oleic acid and also in oily mixture containing oleic acid but oleic acid gets decomposed due to air oxidation on exposure to atmospheric condition (Ainley Wades & Paul J Waller., 1994). Hence oleic acid was not used in the present study. Ethyl oleate shows next highest solubility in the series and also in oily mixture containing tween-80 and PG. Solubility of valdecoxib in oily mixture increases due to the solubilization effect of tween-80 and further PG reduces the interfacial tension which helps in solubilization of valdecoxib in oily phase. Hence, ethyl oleate was used as oil along with tween-80 as surfactant for development of o/w type microemulsion and PG as co-surfactant, which helps in adjusting HLB value of formulation. The pseudoternery phase diagrams with various weight ratios of tween-80: PG is depicted in Figure 1. The transparent microemulsion region was observed visually and also by using cross polarizer. Only those systems, which appeared black when visualized through cross polarizer, were deemed to be within the microemulsion region. The rest of the region on the phase diagram represents turbid and conventional emulsion. The area of microemulsion region changed slightly in size in the order of 3:1>2:1>1:1 with the increasing ratio of tween-80 to PG. Various microemulsions were selected from 1:1, 2:1 and 3:1 phase diagrams and the drug was incorporated. The various characterization parameters of microemulsions aredepicted in Table 2. The optical birefringence studies suggested that all the microemulsions were found to be isotropically clear. All the microemulsions had small average droplet diameters between 12.4-15.3 nm. The droplet size was found to be increase significantly (P<0.5) with more oil as in MA and MB series, which can be attributed to expansion of oil drop of microemulsion and also due to the variation in phase volume ratio of oil to water during emulsification. In MC series the droplet size was small which may be due to large amount of surfactant. From overall result the microemulsions containing 3:1 ratio of tween-80 to PG shows small droplet size. Small droplet size was preferred in term of skin penetration. The polydispersity index was 0.120 – 0.858 for all microemulsions indicating homogeneity in globule size distribution. All the microemulsions showed high conductivity values of 82.80-249.47 μs/cm suggesting that all microemulsions were o/w type. All the prepared microemulsions were then incorporated in 0.75% w/w carbopol 940 to obtain emulgels as shown in Table 3. All the emulgels were clear and transparent in appearance. The various evaluation parameters of emulgels are represented in Table 4. The pH of all emulgels was found between 5.0-5.5, which lies in the normal pH range of the skin. All the prepared emulgel formulations showed uniformity in drug content and were within permissible range indicating the uniformity of the drug dispersion in the emulgels. All formulated valdecoxib emulgels showed an increase in the viscosity as the amount of oil was increased and also the proportion of tween-80 increased. The increase in viscosity was due to decrease in the aqueous phase of emulgel. In the skin irritation study as represented in Table 5, the emulgel formulations showed no skin irritation even after 72 hours with zero scoring for erythema which indicates better skin acceptability for topical applications. The in vitro permeation of valdecoxib from different emulgels was affected by many parameters like globule size, viscosity, amount of oil and proportion of tween-80 to PG. The percent drug release from all emulgels is shown in Fig2,3&4. In MA series of emulgels, the percent drug release was decreasing in the order of MA1>MA2>MA3 which may be due to the decrease in the water content in the formulation and also increase in the viscosity.
In MB series of emulgels, MB1 showed maximum percent drug release. The reason is same as that of MA series. The MC series showed highest percent drug release as compared to MA and MB series which was very high as compared to MA and MB series. This was due to highest proportion of tween-80 as a surfactant, which acts as a permeation enhancer, and also the globule size was less compared to MA and MB series and hence small globule size was preferred in terms of skin penetration. The viscosity of MC series was high as compared to MA and MB series but due to the reasons said above, the release was more. The overall in vitro release was in the order of MC series > MB series> MA series. All the formulations obeyed Higuchi’s equation suggesting diffusion controlled release mechanism and the release kinetics was first order.
From the study it can be concluded that microemulsion based emulgel formulation containing Tween-80: PG in the proportion of 3:1 was the best formulation. Hence microemulsion based gels could be successfully used as a vehicle for topical delivery of Valdecoxib.
Acknowledgement
The authors are thankful to the Head, Department of Pharmaceutics, for providing laboratory facility and also to Arti Drugs Ltd. Mumbai for providing gift sample of drug.
References
- Peltola, S., Saarinen-Savolainen, P., Kiesvaara, J., Suhonen, T.M., & Urtti, A. (2003). Microemulsions for topical delivery of estradiol. J. Pharm., 254, 99–107.
- Huabing Chen et al. Microemulsion-based hydrogel formulation of ibuprofen for topical delivery. International Journal of Pharmaceutics. 2006; 315: 52-58.
- Delgado-Charro, M. B., Iglesias-Vilas, G., Blanco-Mendez, J., Lopez-Quintela, M. A., Marty, J. P., & Guy, R. H. (1997). Delivery of a hydrophilic solute through the skin from novel microemulsion system. J. Pharm. Biopharm. 43, 37–42.
- Djordjevic, L., Primorac, M., Stupar, M., & Krajisnik, D. (2004). Characterization of caprylocaproyl macrogolglycerides based microemulsion drug delivery vehicles for an amphiphilic drug. J. Pharm., 271, 11-19.
- Gondaliya, D.P., & Pundarikakshudu, K. (2002). Studies on preparation, characterization and transdermal permeation of nimesulide from aqueous and emulgel. Indian Drugs., 39 (9), 465-73.
- Kreilgaard, M. (2002). Influence of microemulsions on cutaneous drug delivery. Drug Deliv. Rev., 54(1), 77-98.
- Kashappa Goud, Desai H. Enhanced skin permeation of rofecoxib using topical microemulsion gel. Drug Development Research. 2004; 63(1): 33-40.







