S. Girija and V. Y . Rajalakhsmi
Department of Plant Biology and Biotechnology, Ethiraj college for women, Chennai.
Abstract
Momordica charantia Linn. belongs to the family cucurbitaceae. It is a medicinal plant used for many diseases. It is a climbing annual or perennial herb is used as vegetable. I have studied its phytotoxic effect in both in vitro and in vivo analyses. It was studied by using the ethanolic extract of leaf and fruit of Momordica charantia on common weeds like Commelina and Aerva by leaf disc assay. Leaf discs of Commelina and Aerva were incubated for 24 and 48 hrs in the ethanolic extract. The result shown remarkable reduction in the chlorophyll content. These result suggest that the fruit and leaf extract of Momordica charantia possesses the phytotoxic effect.
Keywords
Phytotoxicity; Ethanolic extract; phytochemical analysis; secondary metabolites
Download this article as:| Copy the following to cite this article: Girija S , Rajalakhsmi V. Y .Phytotoxic Effect of Momordica Charantia on Common Weeds (Commelina & Aerva). Biomed Pharmacol J 2008;1(1). |
| Copy the following to cite this URL: Girija S , Rajalakhsmi V. Y .Phytotoxic Effect of Momordica Charantia on Common Weeds (Commelina & Aerva). Biomed Pharmacol J 2008;1(1). Available from: http://biomedpharmajournal.org/?p=230 |
Introduction
Medicinal plants are the local heritage with global importance, world is endowed with a rich wealth of medicinal plants. Herbs have always been the principal form of medicine in India and presently they are becoming popular throughout the developed world, as people strive to stay healthy in the face of chronic stress and pollution and to treat illness with medicine that work in concert with the body’s own defense. People in Eroupe, North America and Australia are consulting trained herbal professionals and are using the plant medicines. Medicinal plants also play an important role in the lives of rural people, particularly in remote parts of developing countries with few health facilities.
In India medicinal plants have a good contribution to the development of ancient Indian Material medica. One of the earliest treaties on Indian medicine, the charka smite(1000 B.C),records the use of over 340 drugs of vegetable origin, most of these continue to be gathered from wild plants to meet the demand of the medical profession.
The subcontinent, India is blessed with varieties of aromatic and medicinal plants, the agro climatic conditions and rainfall favoring this bio-availability. More than 7,500sp of medicinal plants are grown in India. Owing to India is considered as the botanical garden of the world and treasure house of the biodiversity.Ayurveda, our indigenous system of health care is accepted everywhere especially abroad. Vedas and other ancient scriptures give clean out evidence of using herbs and medicinal plants. Ayurveda alone describes about 2000sp of plants, which contribute more than 10000 formulations. (Orient Longman, 1997)
Momordica charantia .Linn. belongs to the family cucurbitaceae. It is a medicinal plant used for many diseases. It has been extensively studied as a traditional treatment for diabetes. It is a climbing annual or perennial herb is used as vegetable indigenous to tropical areas including India, Asia, South America and Africa. Various preparation of Momordica charantia from extracts of fruit juices and dried fruits have been used world wide particularly for blood sugar lowering effect (Raman & Lau 1996)
Momordica charantia is used in preparation of drug in Ayurveda, Unani system of medicine. The roots of M.charantia are used in ophthalmic and prolapsus vaginae. The fruit is bitter, cooling, digestable, laxative, antipyretic, anthelmintic, and appetizer. It is used in anaemia, urinary disorders, asthma, ulcers and bronchitis. The juice is useful in cholera. It also has various medicinal properties such as carminative, tonic, stomachic, aphrodisiac, anthelmintic, astringent to the bowels and expectorant. The leaves are given for bilious affection as an emetic and purgative. It also useful in piles, leprosy, jaundice and as a vermifuge. It also used as emmenogoue in dysmenorhoea. The fruit is much value in stomachic (Natkarni 1991).
In addition it as been reported to exhibit diverse biological activities such as antioxidant, antimicrobial, antiviral, antihepatotoxic, antiulcerogenic activity which is attributed to an array of biologically active plant chemical including triterpenes, pisteins and steroid (Grover&Yadav 2004). It also consists of many chemical constituents such as alkaloid, alkaline, ascorbic acid, asparagines, aspartic acid, citrulline, cucurbitane, and momordicine etc,. Momordica charantia are frequently used in folk medicine. M.charantia has show insecticidal activity and also has antiplasmodial property. Hence M.charantia is tested for their phytotoxic effect on common weeds like Commelina and Aerva.
The objective of the present study.
Extraction of dried leaf and fruit of M.charantia.
Study of phytotoxic effect on Commelina sp and Aerva sp.
Phytochemical analysis of ethanolic extract.
Three new cucurbitane type triterpene called karavilagenis A, B, C and five new cucurbitane type triterpene glycosides called karavilosides were isolated from dried fruit of M.charantia together with two cucurbitane type triterpene 19,5,19-epoxycucurbita goyaglycosides-b,c&d, momordicosides (Nakumara et al 2007)
Investigation of the traditional uses of Momordica charantia (cucurbitaceae) in Togo (West Africa) showed that it is one of the most important local medicinal plants both for ritual and ethnomedical practices. There was a high degree of consensus (>50%) for use in the treatment of gastrointestinal and viral diseases among general population. M.charantia extract prepared from accessions collected in Togo showed high antiviral activity (<5µg/ml) against sindbis and herper simplex type 1 viruses and anthelmintic activity against caenorhabdites elegans. Momordicins were found. To be anthelmintic but not antiviral (Nadine belonin at al 2005).
The phytochemical screening was performed by Standard methods. Estimation of proteins was done by Lowry’s method. Aminoacid were separated and identified by chromatographic method. Among the species studied the highest protein content was found in the pollen grains. 16 Aminoacid were identified in the pollen grains of cucurbitaceae plant investigated (Kalkal et al 2005).
Scanning electron microscopic studies on the effect of treatment with alcohol extract of M.charantia seeds to male albino rat at a dose of 25mg / 100g body weight orally for 35 days on the cauda epididymal sperms indicated that plasma membrane and acrosomal membrane are distributed with serration in the sperm head region. Considerable change in the shape and size of sperm exhibited abnormality. There was appearance of cytoplasmic droplets in the mid tail region (Girni et al 2005).
The protein provides along with other ingredient suitable formulation in the tablet form in the treatment of diabetes mellitus (Khanna et al 2006).
A significant decrease in malondialdehyde (MDA) level and a significant increase in antioxidant activity were observed when M.charantia, Allium sativum, Azardiracta indica and Ocimum sanctum were administered to diabetic rats. Changes in metal ion status were also observed following administration of herbal hypoglycemic agents. It is concluded that these herbal drug also decreases the oxidative load and strength the antioxidant potential (Chandra, Mahdi 2004) .
M.charantia with exercise reduced the blood glucose ok kk-Ay mice 5 weeks after and administration and also significantly lowered the plasma insulin of kk-Ay mice under similar condition. The blood glucose of M.charantia with exercise is lower than that of M.charantia exercise only 5 weeks after the administration. M.charantia with exercise decreased blood glucose in a glucose tolerance test. These results suggest that M.charantia with exercise is useful for type 2 diabetic cures.
An HPTLC method was developed for quantitative estimation of charatin in small fig. Dried fruits of M.charantia used in formulation and different marketed antidiabetic poly herbal formulation. This HPTLC method was found to be reproducible, accurate and precure and charatin concentration at nanogram .the developed HPTLC method would be important tool in the quality control method of polyherbal formulation (Patel, Goyal 2007).
Macrocyclic trichothene toxin produced by Verrucaria (a phytopathogen of interest in biological weed control) and the non trichothene toxin atronone β from stachybotiys atra were tested for phytotoxicity in duck weed (Lemna pausicostata.L) Plant let culture and Kudzu (Pueraria labata.L). Leaf disc assay for mammalian cytotoxicity in four cultured cell lines. Roridine E and H, epiisororidineE, and Verrucarin A and J were phytotoxic (half maximal effect in the concentration range 0.1-9.7µM on duck weed and 1.5->80µM on kudzu) and cytotoxic to mammalian cell lines (half maximal inhibition of proliferation in the concentration range 1-30µM). Trichoverins A and B and atranoneB were moderately phytotoxic half maximal effect in the concentration range 19-69µM on duck weed and 13 – >80µM on kudzu.
Materials and Method
Preparation Of Plant Materials
Momordica charantia.L. Known as a bitter melon. It is a climbing annual or perennial herb and it is a vegetable crop. It was collected in the month of October –November. Leaves and fruits were dried in shady place and powdered.
Extraction of Plant Material
The fine powdered of the plant material was soaked in ethanol. This was carried out for about three to four days and the solvent is extracted using soxhlet extraction apparatus. At the end of the extraction process the solvent was evaporated and the remaining residue was measured using the formula,
Percentage of yield =weight of dried extract X 100
Weight of dry powder
Analysis Of Phytotoxic Effect (leaf disc method) Invitro
Leaves of both the plants (Commelina and Aerva) were collected freshly and washed with distilled water. Leaf discs are prepared using a sterile cork borer .the five leaf discs were dropped uniformly in all different concentration. The leaf discs weighed (0.076mg) and size (5-10mm) diameters were taken approximately. The leaf extract concentration were ranging from (1000µg, 1200 µg, 1400 µg, 1600 µg, 1800 µg, 2000 µg ) . Leaf disc assay also tried in lower concentration of (100 µg – 1000 µg)
To reduce the phytotoxic effect of the solvent, the extract dilution was prepared in 9:1 ratio with sterile distilled water: ethanol. The two sets of leaf discs were incubated in room temperature for about 24hrs and 48hrs duration. One was maintained as control .The incubation was done for both the plants. Total chlorophyll concentration (Arnon’s method) was checked after 24hrs incubation and 48hrs incubation in both the plants. (Plate 2, 3) The leaves were removed and gently rinsed in distilled water. The same procedure was repeated for fruit extract also.
After the incubation of 24hrs and 48hrs the leaf discs were taken out and blot it with filter paper. The leaf discs were weighed and ground using ethanolic solvent and made up to known volume (10ml). This was read at 645nm and 663nm using a spectrophotometer.
The Rf value was calculated using the formula,
Total chlorophyll (mg/ml) = [(20.2*OD 645) + (8.02*OD663)] x V/a x 1000x W
Where,
a = length of the light path in the cuvette (1cm)
V = volume of the extract in ml (10ml)
W= weight of the leaf disc in mg
In vivo Method
Similar type of tests was conducted on the same plants (Commelina and Aerva) by spray method. The plants were grown in pots. Various concentration of leaf extracts ranging from (1200 µg, 1400 µg, 1600 µg, 1800 µg, 2000 µg) were sprayed periodically in sterile condition. These plants were tested for total chlorophyll concentration by Arnon’s method. The same procedure was repeated with the fruit extract also. (Plate 4&5)
Thin Layer Chromatography
A crude ethanolic extract was spotted on a precoated silica gel TLC plate. The spot were allowed to dry. The plates were developed in a developing tank containing Toluene: Hexane: Ethanol in3:3:1 ratio. The chromatogram was developed for about 15mins. The Rf values of different coloured spots were calculated.
The Rf value were calculated using the formula,
Rf = solute front/ solvent front
Phytochemical Tests
The ethanolic extract of leaf and fruit was tested for the metabolites by the following phytochemical tests. It also tested with eluted bands.
Alkaloid
Potassium iodide and Iodine was added to the 1 µg of extract and observed red precipitate or pink layer at the top
Cardiac glycoside
To 1 µg of extract 2ml of glacial acetic acid, 1 drop of ferric chloride, 1ml of concentrated sulphuric acid was added and observed for brown colour formation between 2 layers or violet ring or green ring may be present.
Flavanoid
To 1 µg of extract, 5ml of dilute Ammonium solution and concentrated sulphuric acid was added and observed for yellow colour or observed for the disappearance of yellow colour.
Phlobatannin
To 1µg of the extract, 1% Hcl was added and observed for red precipitation.
Saponin
To 1 µg of the extract, 2ml of H2O was added and shaken vigorously till persistent froth was observed.
Steroid
To 1 µg of the extract, 2ml of Acetic anhydride and 2ml of concentrated sulphuric acid was added and observed for violet or blue or green colour.
Tannin
To 1 µg of the extract few drops of 1% ferric chloride was added and observed for brownish green or blue black colour.
Terpenoid
To 1 µg of the extract, 2ml of chloroform and concentrated sulphuric acid was added and observed for reddish brown interference.
Leaf Disc Assay In Eluted Bands
The leaf discs of Commelina was incubated in eluted bands for 24 hrs and observed for its chlorophyll content.
Result and Discussion
The leaf and fruit ethanolic extract of the plant sample was weighed. The crude extract of the sample of leaf was 1.17 mg and fruit was 1.20 mg was collected and stored in an air tight container separately.
Phytotoxic effect – Invitro Analyses
The leaf and fruit extract was tested on two different plants (Commelina and Aerva) showed the following results. The leaf disc assay of commelina sp showed remarkable difference between 24hrs and 48hrs of incubation. The results were tabulated (table 1 &2).
The total chlorophyll content was expressed in percentage. The percentage of the chlorophyll content was calculated using chlorophyll concentration of control as standard value. There was a marked reduction in the chlorophyll content where the concentrations of the plant extract were increase.
The concentration of the leaf extract on Commelina sp showed remarkable reduction in chlorophyll content (1200 µg –22.59%, 1400 µg – 29.04% , 1600 µg – 33.34%, 1800 µg –35.49% , 2000 µg –37.64%) . were recorded after 24hrs( table– 1) and (1200 µg – 13.68 %, 1400 µg– 15.78% , 1600 µg –20.00%, 1800 µg–24.21%, 2000 µg– 28.42%) were recorded for 48hrs (table–3).
The concentration of the fruit extract also showed remarkable reduction in chlorophyll content. It was (1200 µg–26.32%, 1400 µg– 33.67%, 1600 µg– 38.96%, 1800 µg–42.11%, 2000 µg– 49.48%) after 24hrs(table 1) and ( 1200 µg– 27.64%, 1400 µg– 37.70%, 1600 µg– 50.81%,1800 µg–51.63%, 2000 µg–55.74%) after 48hrs (table 2).
Therefore fruit extract of M.charantia showed distinct change in chlorophyll content than leaf extract. The tabulated result on the whole showed that fruit extract was more phytotoxic than that of leaf extract on the tested plant Commelina sp.(fig 1 &2)
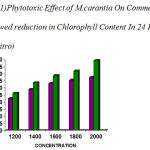 |
Figure 1: Phytotoxic Effect of M.carantia On Commelina Showed reduction in Chlorophyll Content In 24 Hrs (Invitro)
|
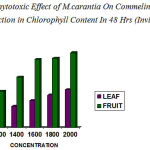 |
Figure 2: Phytotoxic Effect of M.carantia On Commelina Showed Reduction in Chlorophyll Content In 48 Hrs (Invitro)
|
The leaf disc assay of Aerva sp also showed some difference in chlorophyll between 24hrs and 48hrs of incubation. The results were tabulated. The leaf extract showed ( 1200 µg– 47.62%,1400 µg– 26.20%, 1600 µg– 23.81%,1800 µg– 26.20%, 2000 µg– 26.20%) for 24hrs respectively, and for 48hrs (1200 µg–25.00%, 1400 µg– 27.00% , 1600 µg–29.50%, 1800 µg– 25%, 2000 µg– 25%) respectively (table 3 &4).
The concentration of the fruit extract showed reduction in chlorophyll content was higher in 48hrs than 24hrs incubation (table 3&4).the fruit extract showed remarkable reduction than leaf extract (fig 3&4).
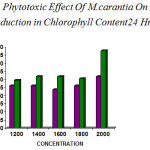 |
Figure 3: Phytotoxic Effect Of M.carantia On Aerva sp Showed reduction in Chlorophyll Content24 Hrs (Invitro)
|
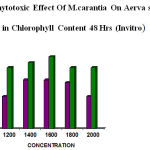 |
Figure 4: Phytotoxic Effect Of M.carantia On Aerva sp Showed reduction in Chlorophyll Content 48 Hrs (Invitro)
|
However the plan extract showed lower inhibitory effect on Aerva than Commelina. The reduction percentage of chlorophyll content in Aerva reasonably lower with that of commelina sp.
In vivo Analysis
The phytotoxic effect of plant extract on Commelina sp and Aerva sp was confirmed using spray method and the respective concentration were recorded by leaf disc assay. The leaf disc assay of Commelina sp showed remarkable difference between 24hrs and 48hrs incubation. The results were tabulated.
The total chlorophyll content was expressed in percentage. The percentage of the chlorophyll content was calculated using chlorophyll concentration of control as standard value. There was a marked reduction in chlorophyll content were the concentration of the plant extract were increased.
The concentration of the leaf extract on Commelina sp showed (1200µg– 22.22%, 1400µg– 24.44%, 1600µg– 33.30%, 1800µg– 40.00%, 2000µg– 46.60%) for 24hrs and (1200µg– 20.45%, 1400µg– 29.54%, 1600µg– 34.09%, 1800µg–40.90%, 2000µg–50%) for 48hrs reading.
The concentration of fruit extract also showed reduction in chlorophyll content (1200 µg– 24.21% , 1400µg– 31.57%, 1600µg–36.84%, 1800µg– 43.15%, 2000µg– 49.47%) for 24hrs and (1200µg– 22.58%, 1400µg– 41.93%, 1600µg– 51.61,1800µg– 63.44%,2000µg–68.81%) for 48hrs observation.(table 5&6)
Thus the fruit showed remarkable chlorophyll reduction than leaf extract . (fig 5&6)
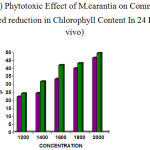 |
Figure 5: Phytotoxic Effect of M.carantia on Commelina Showed reduction in Chlorophyll Content In 24 Hrs (In vivo)
|
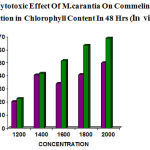 |
Figure 6: Phytotoxic Effect Of M.carantia On Commelina Showed reduction in Chlorophyll Content In 48 Hrs (In vivo)
|
The similar type of work was conducted in Aerva sp also. The results for 24hrs showed (1200µg–18.18%, 1400µg– 15.9%, 1600µg– 10.00%, 1800µg– 15.9%, 2000µg–18.18%) and for 48 hrs (1200µg–17.64%, 1400µg– 16.47%, 1600µg–17.64%, 1800µg–17.64%, 2000µg–25.68%) respectively. The concentration of fruit extract also showed remarkable reduction in chlorophyll content (table 7&8). The fruit extract showed higher phytotoxic effect than leaf extract (fig 7&8)
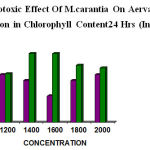 |
Figure 7: Phytotoxic Effect Of M.carantia On Aerva sp Showed reduction in Chlorophyll Content24 Hrs (Invivo)
|
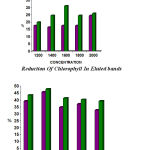 |
Figure 8: Phytotoxic Effect Of M.carantia On Aerva sp Showed reduction in Chlorophyll Content 48 Hrs (Invivo)
|
However the plant extracts lower inhibitory effect on Aerva sp than on Commelina. The reduction % of the chlorophyll content of Aerva sp relatively lower than that of Commelina sp.
Phytochemical Analysis
The phytochemical analysis of aqueous fruit and leaf extract showed the presence of flavanoid, alkaloid, cardiac glycoside, steroid and terpenoids (table 9)
Chromatographic Analysis
The developed chromatogram showed different bands. The Rf values for different bands were tabulated (table 10).
Phytochemical Analysis of Eluted Bands
The bands which are eluted from chromatogram showed some positive result in phytochemical analysis. It shows the presence of alkaloid, flavanoid, steroid and terpenoid (table 11)
Leaf Disc Assay In Eluted Bands
Leaf disc assay in eluted bands showed remarkable reduction in the chlorophyll content in various bands (table 12).
Phytotoxic Effect of Momordica charantia On Commelina sp (Invitro)
Table 1: 24 Hours
|
CONCENTRATION |
LEAF |
FRUIT |
||
| %of total chlorophyll | Reduction in chlorophyll content | %of total chlorophyll content | Reduction in chlorophyll content | |
| Control | 100 | 0 | 100 | 0 |
| 1200 | 77.4 | 22.59 | 73.68 | 26.32 |
| 1400 | 70.96 | 29.04 | 66.31 | 33.69 |
| 1600 | 66.66 | 33.34 | 61.05 | 38.96 |
| 1800 | 64.51 | 35.49 | 57.89 | 42.11 |
| 2000 | 62.36 | 37.64 | 50.52 | 49.48 |
Table 2: 48 Hours
|
CONCENTRATION |
LEAF |
FRUIT |
||
| %of total chlorophyll | Reduction in chlorophyll content | %of total chlorophyll content | Reduction in chlorophyll content | |
| Control | 100 | 0 | 100 | 0 |
| 1200 | 86.31 | 13.68 | 72.95 | 27.04 |
| 1400 | 84.21 | 15.78 | 62.30 | 37.70 |
| 1600 | 80.00 | 20.00 | 49.18 | 50.81 |
| 1800 | 75.78 | 24.21 | 48.36 | 51.63 |
| 2000 | 71.57 | 28.42 | 44.26 | 55.74 |
Phytotoxic Effect Of Momordica charantia Of Aerva sp (Invitro)
Table 3: 24 Hours
|
CONCENTRATION |
LEAF |
FRUIT |
||
| %of total chlorophyll | Reduction in chlorophyll content | %of total chlorophyll content | Reduction in chlorophyll content | |
| Control | 100 | 0 | 100 | 0 |
| 1200 | 73.80 | 26.20 | 70.45 | 29.54 |
| 1400 | 73.80 | 26.20 | 68.18 | 31.82 |
| 1600 | 76.19 | 23.81 | 68.18 | 31.82 |
| 1800 | 73.80 | 26.20 | 69.31 | 30.68 |
| 2000 | 52.38 | 31.82 | 68.18 | 47.62 |
Table 4: 48 Hours
|
CONCENTRATION |
LEAF |
FRUIT |
||
| %of total chlorophyll | Reduction in chlorophyll content | %of total chlorophyll content | Reduction in chlorophyll content | |
| Control | 100 | 0 | 100 | 0 |
| 1200 | 86.66 | 13.33 | 75 | 25 |
| 1400 | 80 | 20 | 72.72 | 27 |
| 1600 | 80 | 20 | 70.45 | 29.5 |
| 1800 | 81.11 | 18.88 | 75 | 25 |
| 2000 | 86.66 | 13.33 | 75 | 25 |
Phytotoxic Effect of Momordica charantia On Commelina sp (Invivo)
Table 5: 24 Hours
|
CONCENTRATION |
LEAF |
FRUIT |
||
| %of total chlorophyll | Reduction in chlorophyll content | %of total chlorophyll content | Reduction in chlorophyll content | |
| Control | 100 | 0 | 100 | 0 |
| 1200 | 77.77 | 22.22 | 75.78 | 24.21 |
| 1400 | 75.55 | 24.44 | 68.42 | 31.57 |
| 1600 | 66.66 | 33.33 | 58.02 | 41.98 |
| 1800 | 60.00 | 40.00 | 56.84 | 43.15 |
| 2000 | 53.33 | 46.60
|
50.52 | 49.47 |
Table 6: 48 Hours
|
CONCENTRATION |
LEAF |
FRUIT |
||
| %of total chlorophyll | Reduction in chlorophyll content | %of total chlorophyll content | Reduction in chlorophyll content | |
| Control
1200 1400 1600 1800 2000 |
100
79.54 70.45 65.90 59.09 50.00 |
0
20.45 29.54 34.09 40.90 50.00 |
100
77.41 58.06 48.38 36.55 31.18
|
0
22.58 41.93 51.61 63.44 68.81 |
Phytotoxic Effect Of Momordica charantia of Aerva sp (In vivo)
Table 7: 24 Hours
|
CONCENTRATION |
LEAF |
FRUIT |
||
| %of total chlorophyll | Reduction in chlorophyll content | %of total chlorophyll content | Reduction in chlorophyll content | |
| Control
1200 1400 1600 1800 2000 |
100
81.81 84.09 90.00 84.09 81.81 |
0
18.18 15.90 10.00 15.90 18.18 |
100
81.52 73.91 73.91 78.26 79.34
|
0
18.47 26.08 26.08 21.73 20.65 |
Table 8: 48 Hours
|
CONCENTRATION |
LEAF |
FRUIT |
||
| %of total chlorophyll | Reduction in chlorophyll content | %of total chlorophyll content | Reduction in chlorophyll content | |
| Control
1200 1400 1600 1800 2000 |
100
82.35 83.52 82.35 82.35 75.55 |
0
17.64 16.47 17.64 17.64 24.44 |
100
80.00 75.55 68.88 75.55 74.11 |
0
20.00 24.44 31.11 24.44 25.89
|
Phytochemical Analysis on Plant extract
Table 9
|
S.NO |
SECONDARY METABOLITE |
RESULTS |
|
1
2
3
4
5
6
7
8
9
10
11 |
Alkaloid
Anthraquinone
Cardiac glycoside
Flavanoid
Phenol
Phytosterol
Phlobatannins
Sapponin
Steroid
Tannin
Terpenoids |
+
_
+
+
_
_
_
_
+
_
+ |
Rf Values Of Different Bands
Table 10
|
S.NO |
BANDS |
SOLUTE FRONT |
SOLVENT FRONT |
RF VALUE |
|
1
2
3
4
5 |
Band 1
Band 2
Band 3
Band 4
Band 5 |
2
3.5
4.1
6.8
9.4 |
9.8
9.8
9.8
9.8
9.8 |
0.20
0.35
0.41
0.69
0.95 |
Phytochemical Test in Eluted Bands(Tab-11)
|
S.NO |
SECONDARY METABOLITE |
BANDS | ||||
| 1 | 2 | 3 | 4 | 5 | ||
| 1
2
3
4
5 |
Tannin
Steroid
Alkaloid
Flavanoid
Terpenoid |
_
_
_
_
+ |
_
_
+
_
_ |
_
_
_
+
+ |
_
+
_
_
_ |
_
_
_
_
_ |
Leaf Disc Assay In Eluted Bands
|
S.NO |
BANDS |
LEAF |
FRUIT |
||
| %of total chlorophyll | Reduction in chlorophyll content | %of total chlorophyll content | Reduction in chlorophyll content | ||
|
1
2
3
4
5
|
Band 1
Band 2
Band 3
Band 4
Band 5
|
60.86
54.34
65.21
63.04
67.39
|
39.14
45.65
34.78
36.99
32.60
|
56.52
52.17
58.69
59.78
60.86
|
43.47
47.82
41.30
40.21
39.13
|
Conclusion
During the course of this study the phytotoxic effect of crude extract of Momordica charantia was high in fruit extract than that of leaf extract sample. The effect of fruit and leaf extract on Commelina sp was high when compared to that of Aerva sp.
It was also noted that secondary metabolites like flavanoids, steroid and terpenoid content were high in both leaf and fruit extract. It was also understood that these secondary metabolites distinctly showed specific phytotoxic effect.
Finally, the analysis brings out certain substances present in crude extract sample like sapponin, alkaloid show principle naturally stored in plant organ. Further quantification and identification could impart more information.
Reference
- AbbasHK, GelderblomWCA, CawoodME, ShierWT, (1993).Biological activities of famonisins mycotoxin from Fusarium moniliform in Jimson weed (Datura stramonium .L.)And mamalian cell cultures. Toxicon. 31: 343-353
- Abbas HK, Tak, Boyette CP, ShierWTand Jaruis BB, (2001).Macrocyclic trichothenes are undetectable in kudzu (pueraria Montana) plants treated with a high producing isolate of Myrothecium venircaria.Phytochemistry. 58: 269-276.
- Abbas H K, Seo, J A, Lee.YW, Musser S M, (1998), Phytotoxic and Cytotoxic of the fumonisin C and P series of Mycotoxin from Fusarium sp fungi. Toxicon 36, 2033-2037
- Agarwl M, kamal R (2007) studies on flavanoid production using in vitro culture of M.charantia .L. Indian Journal of Biotechnology 6(2); 277-279
- Agarwal M, Rakakamal. (2004) studies on steroid production using invitro cultures of M.charantia .journal of medicinal plant science .26; 318-323
- Balandrin MF, kjocke AJ, Wurtele. (1985) Natural plant chemicals; source of industrial and mechanical materials,. Science .228: 1154-1160
- Beloin M, Gbeassor M, Akpagana k, Komlan de soussa, Koumaglo, Arnason. (2005) Ethno medicinal uses of M.charantia in Togo and relation to its phytochemistry. 96; 49-55
- Chopra RN, Nayar SL, Chopra IC. Glossary of Indian, Medicinal plants. New Delhi: Publication and Information Directorate, Council of Scientific and Industrial Research1992; p. 168.
- Chowdhury BL, Bhagora FS, Kichi YS. (2006) plants used by tribal for the control of diabetes in some villages of Banswara district. India. Plant archives 6(2): 785-786
- Girini MM, Ah.med RN, Ahmed M (2004) .The effect of M.charantia seed on rat spermatozoa. Aryavaidyan. 17; 149-153
- GroverJk,RathiSS, YadavSP(2003)assessment of hypoglycemic, antihypoglycemic activities of certain plants.25;10-12
- Grover JK, Yadav SP (2004). Pharmacological actions and potential uses of Momordica charantia: a review. J. Ethnopharmacol. 93: 123-132.
- Gupta C, Rizai G, Panwar M S, Khare A. (2004). composition of M.charantia .L. ethno therapeutic plant. Chemistry biology interface. 2; 26-71
- Hiscox.J.D., Israelstam.G.F, 1979. A method for extraction of chlorophyll from leaf tissue without maceration.can.J.bot.57, 1322-1334
- Kalkar SA, Paulkar N, Mishra A.Phytochemical investigation of protein and amino acid in pollen grains of cucurbitaceae.Trends in plant science. 90; 17-20
- Kiruthika T, Ramachandran S (2003). Hypoglycemic activity of gourd vegetable. Nutrition society of India 35th annual meet.73; 12-13
- Krishnaraju AV, Rao TVN, Sundararaju D. (2005). Assessment of bioactivity of Indian medicinal plants using brine shrimp lethality assay. International J.of applied science.2; 125-134.
- Mathur K, Guryar RBS. (2002) Evaluation of different fungal antagonist, plant extract and against Rhizactonia solani causing stem rot of chilly. Plant protection science .10; 319-322
- Miura T, Itoh, Raman A, Lau C (1996). Anti-diabetic properties and phytochemistry of Momordica charantia L. (Cucurbitaceae). Phytomedicine. 2: 349-362.
- Nakamura S,Murakumi T, Kobayashi H,Yoshikawa, (2006).Chemical and Pharmaceutical Bulletin.54 ; 1545-1550.
- Natkarni K M, Indian material medica, (1991) 4thEdition, popular prakashan, Bombay, vol 1; 805-807
- Patel PM, Patel KN, Patel NM, Goyal RK. Pharmacognosy magazine .2; 224-226
- Welihinda J, Karunanayake EH, Sheriff MH, Jayasinghe KS (1986).Effect of Momordica charantia on the glucose tolerance in maturity onset diabetes. J. Ethnopharmacol. 17: 277-282







