Kishu Tripathi¹ and Shobha Kulshreshtha²
¹Surya College of Pharmacy, Lucknow.
²Head, Department of Pharmacology, SN Medical College, Agra.
Abstract
Metal ions after forming complexes with an antibiotics alters the antimicrorial activity of an antibiotics alone.
Keywords
Metal ions; antibiotics
Download this article as:| Copy the following to cite this article: Tripathi K , Kulshreshtha S. Metalloantibiotics-I. Biomed Pharmacol J 2008;1(1). |
| Copy the following to cite this URL: Tripathi K , Kulshreshtha S. Metalloantibiotics-I. Biomed Pharmacol J 2008;1(1). Available from: http://biomedpharmajournal.org/?p=290 |
Introduction
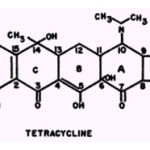
A strong affinity of tetracycline and oxy- and chlortetracycline for metallic ions was reported by Albert (3, 8) who observed the formation of drug-metal complexes of 1:1 and, as the pH is raised, of 2:1. The cations tested (in the order of decreasing stability of the drug-metal complexes) were: Fe++, Al+++, Cu2+ , Ni++, Fe++,Co2+, Zn2+, and Mn2+. The iron complexes of the tetracyclines are red, the Cu2+, and Ni2+ complexes are green, and the Al3+,Co2+,Zn2+, and Mn2+ complexes are yellow. In contrast,Oxford (77) was not able to obtain colored complexes of chlortetracycline with Zn2+or Mn2+but did observe stable yellow complexes with Cu2+, Ni2+, Co2+ Mg2+, Ca2+ and Sr4+.The tetracycline structure contains numerous sites at which chelation with metallic ions might occur. Perhaps the most important sites lie along the system marked by atoms 1 through 7which consists essentially of two 1,3 diketones with two of the keto groups in the enol form.Such monoenols in 1,3 diketones chelate with metallic ions very readily to form six-membered. In each ring, the two atoms that bind the metallic ion are oxygen atoms (23):
 |
Scheme 1 |
 |
Scheme 2 |
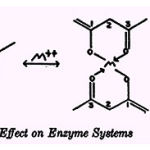
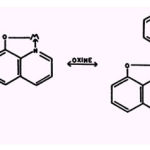
The tetracycline compounds have been described as uncouplers (21) and inhibitors (107)of oxidative phosphorylation and inhibitors of respiration (91, 99, 106), fatty acid oxidation, arginine catabolism (55), nitro reduction (92, 93, 94), and adaptive enzyme formation (15).The possibility that metallic ions might enhance the activity of the drugs was during the series of growth tests described above. Moderate enhancement was observed with low concentrations of Mn2+ or with high concentrations of Fe+ with P. aeruginosa (112,113). Previously, chlortetracycline had been found to be enhanced by similarly low concentrations of Mn+| in its antibiotic effect against C. cucullus (65).The resistant strains tested to date include a strain of M. pyogenes whose average minimum inhibitory concentration of tetracycline and oxy- and chlortetracycline is 120 pg per ml and a strain of Penicillium notatum which requires 750 pug per ml of the drugs for suppression of growth. Fe2+ and Mg2+ are active with the bacterium and Fe+H with the fungus. 8-HYDROXYQUINOLINE Molecular Structure and Affinity for Metallic Cations Of seven isomeric mono-hydroxyquinolines, only 8-hydroxyquinoline (oxine) can chelate metallic ions (9, 90): Sexton (97) observed that the Cu2+, Ni2+ , Cd++, and Ag+compounds of 8-hydroxyquinoline are as fungistatic as oxine itself; and Manten et al. (67)reported that neither Zn+, Cu++, Mn2+ nor
 |
Scheme 3 |
Mo2+-suppresses the toxicity of oxine toward Aspergillus. Other investigators reported that Cations Cu2+ and Fe2+ enhance antifungal activity (10)and that conalbumen suppresses the antibacterial action of 8-hydroxyquinoline (36). Gram positive bacteria are more susceptible to oxine than are gram negative species and trace amounts of Co2+ excess amounts of Fe++ suppress the activity of 8-hydroxyquinoline against the former organisms whereas Fe2+, Zn2+ or Cu2+ suppress the toxicity of oxine towards the latter bacteria. Furthermore, with Micrococcus pyogenes, an increase in the concentration of 8-hydroxyquinoline results in a paradoxical decrease in toxicity. Subsequent studies revealed that small concentrations of Fe2+ Cu2+, or Cd2+ are required for toxicity towards gram positive species and that a 2:1 oxine-Fe- molar ratio is maximally toxic.
References
- Albert, A. 1953 Avidity Of Terramycin And Aureomycin For Metallic Cations.Nature, 172, 201.
- Albert, A., And Rees, C. W. 1956 Avidity Of The Tetracyclines For The Cations Of Metals. Nature, 177, 433-434.
- Albert, A., Rubbo, S. D., Goldacre, R. J.,And Balfour, B. G. 1947 A Study Of 8-Hydroxyquinoline (Oxine) And Related Compounds. Brit. J. Exptl. Pathol., 28, 69-87.
- Bernheim, F. 1954 The Effect Of Certain Metal Ions And Chelating Agents On The Formation Of An Adaptive Enzyme In Pseudomonas Aeruginosa. Enzymologia, 16, 351- 354.
- Brody, T. M., Hurwitz, R., And Bain, J. A. 1954 Magnesium And The Effect Of The Tetracycline Antibiotics On Oxidative Processes In Mitochondria. Antibiotics And Chemotherapy, 4, 864-870.
- Calvin, M., And Wilson, K. W. 1945 Stability Of Chelate Compounds. J. Am.Chem. Soc., 67, 2003-2007.
- Feeney, R. E. 1951 The Antagonistic Activities Of Conalbumin And 8-Hydroxyquinoline (Oxine). Arch. Biochem., 34,196-208.
- Little, P. A., Oleson, J. J., And Williams, J. H. 1953 Factors Influencing The Sensitivity Of Protozoa To Antibiotics. Antibiotics And Chemotherapy, 3, 29-34.
- Manten, A., Klopping, M. L., And Van Der Kerk, G. J. M. 1951 The Influence Of Essential Trace Metals Upon The Fungitoxicity Of Tetramethylthiuram Disulphide And 8-Hydroxyquinoline. Antonie Van Leeuwenhoek. J. Microbiol. Serol., 17, 58-68.
- Oxforid, A. E. 1953 A Colorimetric Method, Based On Metallic Complex Forma- Tion, For The Detection Of Aureomycin In Presence Of Amino Acids And Proteins.Nature, 172, 395-396.
- Rubbo, S. D)., Albert, A., And Gibson, M. I. 1950 The Antibacterial Activity Of8-Hydroxyquinoline (Oxine). Brit. J. Exptl. Pathol., 31, 425-441.
- Santi, R., And Berti, T. 1950 Fenomeni Di Interferenza Tra Elettroliti E Antibiotici Ii. Aureomicina. Arch. Internat. Pharma- Codynamie, 82, 63-66.
- Saz, A. K., Brownell, L. W., And Slie, R. B. 1956 Aureomycin-Resistant Cell- Free Nitro Reductase From Aureomycin- Resistant Escherichia Coli. J. Bacteriol., 71, 421-424.
- Saz, A. K., And Martinez, L. M. 1956 Enzymatic Basis Of Resistance To Aureo-, Mycin. 1. I)Differences Between Flavoprotein Nitro Reductase Of Sensitive And Resistant Escherichia Coli. J. Biol. Chem., 223, 285- 292.
- Saz, A. K., And Slie, R. B. 1953 Manganese Reversal Of Aureomycin Inhibition Of Bacterial Cell-Free Nitro-Reductase. J. Am. Chem. Soc., 75, 4626.
- Saz, A. K., And Slie, R. B. 1954 Reversal Of Aureomycin Inhibition Of Bacterial Cell- Free Nitro Reductase By Manganese. J. Biol. Chem., 210, 407-412.
- Sexton, W. A. 1952 Chemical Constitution And Biological Activity. 2nd Ed. D. Van Nostrand Company, Inc., New York, N. Y.
- Soncin, E. 1953 Fenomeni Di Interferenza Tra Elettroliti E Antibiotici. Iii. Ione Mag- Nesio E Aureomicina, Terramicina, Chloram- Fenicolo. Arch. Internat. Pharmacody- Namie, 94, 346-352.
- Van Meter, J. C., And Oleson, J. J. 1951 Effect Of Aureomycin On The Respiration Of Normal Rat Liver Homogenates. Science, 113, 273.
- Van Meter, J. C., Spector, A., Oleson, J. J., And Williams, J. H. 1952 In Vitro Action Of Aureomycin On Oxidative Phos- Phorylation In Animal Tissues. Proc. Soc. Exptl. Biol. Med., 81, 215-217.
- Weinberg, E. D. 1954 The Influence Of Inorganic Salts On The Activity In Vitro Of Oxytetracycline. Antibiotics And Chemotherapy, 4, 35-42.
- Weinberg, E. D. 1954 The Reversal Of The Toxicity Of Oxytetracycline By Multivalent Cations. J. Infectious Diseases, 95, 291-301.







