Bincy Thomas¹, V. V. Pande², A. R.Tekade³ and K. V. Shastri4
¹Departemnt of Pharmaceutical Analysis, Allana College of Pharmacy, Pune.
²Department of Pharmaceutical Analysis, Jayawantrao Sawant College of Pharmacy and Research, Hadapsar, Pune.
³Departement of Pharmacognosy and Phytochemistry Vivekanand Education Society’s College of Pharmacy Chembur (E), Mumbai.
4Department of Pharmaceutics, R.C. Patel College of Pharmacy, Shirpur.
Abstract
Nifedipine, a calcium channel blocker inhibits the movement of calcium ions across the membranes of cardiac and arterial muscle cells is mainly indicated in angina pectoris, hypertension, raynaud’s phenomenon, myocardial infarction and asthma. It is recommended to administer Nifidipine as modified release dosage form in order to avoid fluctuations in blood vessels. There are several brands of Nifedipine available in the Indian market. Two of such brands of Nifedipine BRAND A and BRAND B were procured from the Indian market and were subjected to spectrophotometric analysis by comparing with the control in order to determine their dissolution patterns. The results indicated that not all the brands of Nifedipine exhibit the same therapeutic effect as there was variation in the release of the active ingredient at the various time intervals and at different levels of pH.
Keywords
Nifedipine sustained release; spectrophotometery; absorbance
Download this article as:| Copy the following to cite this article: Thomas B , Pande V. V , Tekade A. R , Shastri K. V . Comparative Evaluation of Releasing Rate of Nifedipine in S.R. Products from Indian Market by Spectrophotometry. Biomed Pharmacol J 2008;1(1). |
| Copy the following to cite this URL: Thomas B , Pande V. V , Tekade A. R , Shastri K. V . Comparative Evaluation of Releasing Rate of Nifedipine in S.R. Products from Indian Market by Spectrophotometry. Biomed Pharmacol J 2008;1(1). Available from: http://biomedpharmajournal.org/?p=238 |
Introduction
Nifedipine (C17H18N2O6) a dihydropyridine derivative is chemically 3,5 – Pyridine dicarboxylic acid, 1,4- Dihydro-2,6- Dimethyl-4-(2-Nitro phenyl)- Dimethyl ester is indicated for both prophylactic and therapeutic treatment of angina and hypertension. Approximately one quarter of the total global population is affected by at least any one form of the cardiovascular disease (CVD). CVD caused 2.3 million deaths in India in 1990, which may double by year 2020, where hypertension alone contributes for 57% of all stroke death and 24% of all the coronary heart disease. Thus, management of the CVD in particular hypertension becomes important to improve the health care system which demands a sustained release therapy of Nifedipine which will enable to achieve a steady-state tissue level that is therapeutically effective and non-toxic for an extended period of time. There is also a need to design the dosage form so as to optimize the delivery of medication to achieve a measure of control of the therapeutic effect in the face of uncertain fluctuations in the in vivo environment in which drug release takes place. This is usually accomplished by maximizing drug availability i.e. by attempting to attain a maximum rate and extent of drug absorption. Ideally a drug should arrive rapidly at the site of action in the optimum concentration remain for the desired time, be excluded from other sites and be rapidly removed from the sites when indicated. Decreasing the absorption rate and or changing dosage form, provided a useful tool when it is not feasible or desirable to modify the drug compound in a molecular level. However not all the brands of Nifedipine will release same amount and percentage of active ingredient at varied duration of time and pH, thereby revealing their variations in sustained release patterns.
In the present paper, we carried out the comparative evaluation of the releasing rate of Nifedipine in Marketed S.R. Products of different brands i.e. BRAND A and BRAND B along with control by Spectrophotometric Method.
Materials and Methods
Samples of Nifedipine Sustained.Release (S.R.) tablets of different fast moving brands denoted as BRAND A having manufacturing date, september 1996 and expiry date, august 1999 and BRAND B having manufacturing date, january 1997 and expiry date, december 2000 were procured from local Indian market. Dissolution rate on both the brands i.e BRAND A and BRAND B S.R. tablets were determined in order to obtain dissolution pattern of Nifedipine S.R., in different marketed products. The reagents and solvents used were gastric simulated fluid, methanol and distilled water respectively. The instruments employed for performing the study were Dissolution apparatus paddle type (USP No. 1) and Spectrophotometer – Digispec 200GL.
Experimental
From the BRAND A randomly 20 tablets were selected and weighed for calculating their average weight. From it one tablet was selected for studying dissolution rate for which the dissolution apparatus No:1 U.S.P. was used, the paddle was set up at 36 r.p.m. and the temperature was maintained at 37°C. The Dissolution medium contained 720 ml of 0.1 N HCl and 20% methanol i.e. 180 ml to maintain the sink condition. The weighed tablet was put into the flask and the instrument was operated. After one hour the pH is raised to 4.5; and thereafter to 7.2 after three and a half an hour. After that 5 ml of the test solution is taken, at the interval of half an hour upto 8 hours. The samples were taken at regular intervals i.e. for every half an hour. For every removal of the sample, the corresponding buffer was added to maintain the same volume. The sample solution (5 ml) was diluted to 25 ml and the absorbance was measured at 350 nm in Digispec 200GL Spectrophotometer.
The above procedure is repeated in the same manner for BRAND B and control.
Evaluation Methods
Assay of Nifedipine Standard
0.0546 gm of Nifedipine standard sample was dissolved in methanol (50 ml) and from that 2.4 ml was diluted to 50 ml with methanol to give the concentration of 0.0054% w/v solution or 54 microgram per ml. The absorbance was measured at 350 nm.
The amount of released drug in each sample can be calculated by the formulae.
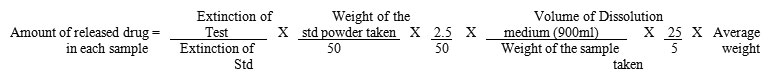
Results
Table 1: Dissolution rate of BRAND A – 20 mg tab
Weight of 20 tablets = 6.2000 gm
Average weight = 0.3100 gm
Weight of tested tablet = 0.3091 gm
| pH | Samples | Time Interval | O.D. | Amount of Drug Released | % of Drug Released |
| 1.2 | I | 30 mins | 0.012 | 3.93 mg | 19.65 |
| II | 30 mins | 0.019 | 6.22 mg | 31.10 | |
|
4.5 |
III | 30 mins | 0.025 | 8.19 mg | 40.95 |
| IV | 30 mins | 0.035 | 11.46 mg | 57.90 | |
| V | 30 mins | 0.038 | 12.44 mg | 62.20 | |
|
7.2 |
VI | 30 mins | 0.040 | 13.10 mg | 65.50 |
| VII | 30 mins | 0.043 | 14.08 mg | 70.40 | |
| VIII | 30 mins | 0.046 | 15.06 mg | 75.30 | |
| IX | 30 mins | 0.050 | 16.38 mg | 81.90 | |
| X | 30 mins | 0.053 | 17.36 mg | 86.50 | |
| XI | 30 mins | 0.055 | 18.01 mg | 90.05 | |
| XII | 30 mins | 0.057 | 18.67 mg | 93.35 | |
| XIII | 30 mins | 0.059 | 19.32 mg | 96.60 | |
| XIV | 30 mins | 0.059 | 19.32 mg | 96.60 |
Table 2: Dissolution rate of BRAND B – 20 mg tab
Weight of 20 tablets = 1.80920 gm
Average weight = 0.09046 gm
Weight of tested tablet = 0.0900 gm
| pH | Samples | Time Interval | O.D. | Amount of Drug Released | % of Drug Released |
| 1.2 | I | 30 mins | 0.020 | 6.58 mg | 32.90 |
| II | 30 mins | 0.023 | 7.57 mg | 37.85 | |
|
4.5 |
III | 30 mins | 0.025 | 8.23 mg | 41.15 |
| IV | 30 mins | 0.028 | 9.21 mg | 46.05 | |
| V | 30 mins | 0.029 | 9.54 mg | 47.70 | |
|
7.2 |
VI | 30 mins | 0.030 | 9.87 mg | 49.35 |
| VII | 30 mins | 0.033 | 10.86 mg | 54.30 | |
| VIII | 30 mins | 0.038 | 12.51 mg | 62.55 | |
| IX | 30 mins | 0.041 | 13.50 mg | 67.50 | |
| X | 30 mins | 0.043 | 14.15 mg | 70.75 | |
| XI | 30 mins | 0.046 | 15.14 mg | 75.70 | |
| XII | 30 mins | 0.050 | 15.80 mg | 79.00 | |
| XIII | 30 mins | 0.048 | 16.46 mg | 82.30 | |
| XIV | 30 mins | 0.053 | 17.45 mg | 87.25 | |
| XV | 30 mins | 0.055 | 18.11 mg | 90.55 | |
| XVI | 30 mins | 0.055 | 18.11 mg | 90.55 |
Table 3: Dissolution Rate of Control – 20 mg tab
Weight of 20 tablets = 4.51540 gm
Average weight = 0.22577 gm
Weight of tested tablet = 0.22515 gm
| pH | Samples | Time Interval | O.D. | Amount of Drug Released | % of Drug Released |
| 1.2 | I | 30 mins | 0.027 | 9.84 mg | 49.20 |
| II | 30 mins | 0.038 | 13.44 mg | 67.20 | |
|
4.5
|
III | 30 mins | 0.047 | 16.39 mg | 81.95 |
| IV | 30 mins | 0.056 | 19.34 mg | 96.70 | |
| V | 30 mins | 0.062 | 21.31 mg | 106.55 | |
| VI | 30 mins | 0.062 | 21.31 mg | 106.55 |
Results and Discussion
The prime objective of sustained release therapy is to achieve a steady state or tissue level that is therapeutically effective and non-toxic for an extended period of time. The rate and extent of release of Nifedipine from SR BRAND A and BRAND B and Control was studied in simulated gastro intestinal fluids for a period of 12 hours. The control tablet (20 mg Nifedipine – which is not Sustained Release) released all its active constituents within 2.5 hrs. BRAND A SR tablet released its active ingredients (4 mg) for minimum effective concentration within half an hour and releasing rate was increased exponentially and about 96.6% of the drug was released after 7 hours. BRAND B SR tablet released its active ingredients (6.5 mg) for minimum effective concentration within half an hour and releasing rate was increased exponentially and about 90.55% of the drug was released after 8 hours.
The graph 1 shows the amount of drug released in mg against time in hours at various pH via 1.2, 4.5, and 7.2. (figure 2.1.)
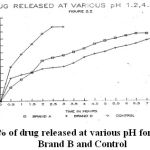 |
Figure 1: % of drug released at various pH for Brand A, Brand B and Control
|
The graph 2 shows the percentage of the drug released against time in hours at various pH via 1.2, 4.5, and 7.2. (figure 2.2.)
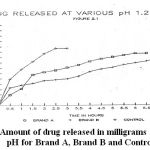 |
Figure 2: Amount of drug released in milligrams at various pH for Brand A, Brand B and Control.
|
Conclusion
The releasing rate of Nifedipine in BRAND B was significant as compared to BRAND A as in BRAND B it was observed that within half an hour the percentage of the drug released was 32.9% giving minimum effective concentration and the rate of release increased slowly i.e. 3 to 5% for every half an hour and about 90.55% of the drug was released after 8 hours whereas in BRAND A 19.65% of the drug was released within half an hour and the rate of release increased 3 to 5% for every half an hour and 96.6% of the drug was released after 7 hours.
Drug release from both the brands of Nifedipine S.R. was almost immediate and thus the fluctuation in blood level during steady state conditions may undermine the sole purpose of the delivery system for Nifedipine though the therapeutic drug blood levels would be maintained for a prolonged period of time. But amongst both the brands, BRAND B S.R. showed desirable blood levels with least fluctuation.
Thus in nutshell, BRAND B S.R. showed therapeutic advantage over BRAND A S.R and control with respect to controlling the fluctuations in plasma levels.
References
- Sorkin, E.M., Clussold, S.P. and Brogden, R.N., Drugs, 1985, 30, 182.
- Heart disease and stroke stastics – 2004 update. American heart association, Dallas, USA, 2004.
- Gupta, R., J. Hum. Hyperten., 2004, 18, 73.
- Panchagnula R, Singh R., Ashokraj Y., In vitro Evaluation of Modified Release Formulations of Nifedipine from Indian Market, Indian Journal of Pharmaceutical Sciences, July-August 2007, 479-608, 556-561.
- Kadam S S, Mahadik K R, Bothra K.G, Principles of Medicinal Chemistry, volume 2, 16th edition reprint October 2006, Nirali Prakashan, Pune, 318-319.
- British Pharmacopoeia 2007, volume 2, The stationary office, Medicines and Healthcare products Regulatory Agency (MHRA), Department of Health, Great Britain, 2006, 1468.
- The United States Pharmacopoeia NF, The National Formulary, Asian Edition, volume 3, 30th revised edition, 2007, 2754-2757.
- Indian Pharmacopoeia 1996, volume 1, Government of India, Ministry of Health and Family Welfare, Controller of Publications, Delhi, 1996, 511-513.
- Remington, The Science and Practice of Pharmacy, 21st edition, volume 2, 1st Indian reprint 2007, Wolters Klower Health (India) Pvt. Ltd, New Delhi,1366.
- Mark H Beers, Robert S Porter, Thomas V Jones, Justin L Kaplan, Micheal Berkwits, The Merck Manual of Diagnosis and Therapy, 18th edition, Merck Research Laboratories, Whitehouse Station, N.J., USA, 613, 2615-2616.
- European Pharmacopoeia, 5th edition, volume 2, Directorate for the Quality of Medicines of the Council of Europe (EDQM), France, 2098-2099.
- The Japanese Pharmacopoeia, 14th edition, Society of Japanese Pharmacopoeia, Tokyo, Japan, 2001, 645-646.
- Handbook of Pharmaceutical Controlled Release Technology, Wise L Donald, 1st Indian reprint 2005, Marcel Dekker Inc, New York, USA, 431-463.
- Robinson R Joseph and Lee H Vincent, Controlled Drug Delivery – Fundamentals and Applications, 2nd edition, revised and expanded, 2nd Indian reprint 2007, Marcel Dekker Inc, New York, USA, 3-61, 253-289.
- Chien W Yie, Novel Drug Delivery Systems, 2nd edition, revised and expanded, 2nd Indian reprint 2007, Marcel Dekker Inc, New York, USA, 1-42.
- Lachman Leon, Lieberman A Herbert, Kanig L Joseph, The Theory and Practice of Industrial Pharmacy, 4th Indian reprint 1991, Varghese Publishing House, Bombay, 430- 456.
- Sean C. Sweetman, Martindale, The Complete Drug Reference, 35th edition, Pharmaceutical Press, London, UK, 2007, 1213-1221.







