Archana P. Ramteke and Mandakini B. Patil*
University Department of Biochemistry, RTM Nagpur University, Nagpur
Abstract
Tridax procumbans leaves stem and calyx lectins were found to agglutinate human erythrocytes. The hemagglutination patterns of the erythrocytes of breast cancer patients (Stage I to IV) were compared with the hemagglutination patterns of erythrocytes of normal persons. A gradual decrease in the hemagglutination units was observed with increase in the stages of cancer.
Keywords
Breast cancer ; Lectins ; Tridax procumbans
Download this article as:| Copy the following to cite this article: Ramteke A. P , Patil M. B. Agglutination Pattern of Erythrocytes of Normal Subjects and Cancer Patients with Lectins of Tridax Procumbans. Biomed Pharmacol J 2008;1(1). |
| Copy the following to cite this URL: Ramteke A. P , Patil M. B. Agglutination Pattern of Erythrocytes of Normal Subjects and Cancer Patients with Lectins of Tridax Procumbans. Biomed Pharmacol J 2008;1(1). Available from: http://biomedpharmajournal.org/?p=349 |
Introduction
Scientists have been investigating the nature of the cell membrane. It is well established that cellular adhesion and interactions are largely dependent on the properties of the surface of erythrocytes.
The nature of the neoplastic erythrocyte surface structure was established with the observation that the erythrocytes of normal person and neoplastic patients differ from each other. The phenomenon of invasion and proliferation of cancer cells may well depend largely on the structure of malignant cell membrane 1, 2,3,4,5. It has been observed that the surface sites containing N – acetyl – D – galactosamine residues interact with the agglutinin 6.
Plant lectins were used to detect the alteration in the erythrocytes as they have the ability to recognize membrane glycoconjugates present on the cell surface, and by manifesting significant changes in hemagglutination patterns reflecting the alteration in the erythrocyte membrane. Brooks and co – workers (1991) found that lectins were extremely specific for identifying cells with the improperly assembled membrane glycoproteins of the breast cancer erythrocytes as compared with the erythrocytes of normal person 7.
The present paper describes the results of hemagglutination pattern of normal erythrocytes compared with the erythrocytes of breast cancer patients (Stage I to IV), treated with stem, leaf and calyx lectins of T. procumbans Linn.
Materials and Methods
Extracts of leaves, stem and calyx of the plant T. procumbans L (Family – Compositeae) were used as the source of lectin for the present study 8. Papain, bovine serum albumin, guar – gum, D – galactose were obtained from Sigma Chemicals St. Louis Mo, USA. Other chemicals were of analytical grade.
Blood samples of normal persons and cancer patients (Stage I to IV) were collected in oxalate bulbs from blood bank, IGGMC and Mayo Hospital, and Rashtra Sant Tukdoji Cancer Hospital, Nagpur.
Preparation of lectins
Leaves, stem and calyx of 45 days old plants were collected from the plants grown in the garden of University Department of Biochemistry, RTM Nagpur University Nagpur, washed four to five times under the tap water and twice with distilled water and soaked between the folds of filter paper and homogenized separately for extraction of lectins as described by Ramteke and Patil, (2005) 9. Lectins were purified by affinity chromatography by the method of Dixon, 1953 10. The purified lectin from leaves was designated at TPL-L, lectin from stem was said to be TPL-S and the lectin of calyx was identified to be TPL-C and characterized as described earlier 9.
Protein Estimation
Protein estimation was carried out by the method of Lowry et al., 1951 using BSA as standard protiens11.
Preparation of Erythrocyte Suspension
The blood samples were washed three times with PBS. The washed erythrocytes were suspended in PBS to prepare a 2% suspension 12.
Agglutination Assay
Hemagglutination assay was carried out using 2% suspension of papain treated erythrocytes of normal subjects and cancer patients. Agglutination inhibition assay was carried out using the method described by Deshpande and Patil, (2002) 13. The titre strength of the lectin was determined as Hemagglutination Units (HAU) taking the reciprocal of the last dilution showing detectable agglutination 14.
Statistical analysis
The hemagglutination activity of erythrocytes of normal persons and breast cancer cases of different cancer stages (Stage I to IV) is compared to student’s t test. The results were statistically analysed {Standard Deviation (Sd), Critical Difference (CD), and Correlation Coefficient (CC)} using the formula as suggested by Persons (1947), 15.
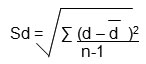
where
Sd = Standard Deviation
d – = The bias (mean difference, d, )
n = number of samples analysed
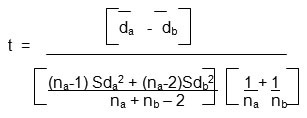
where
t = “Students” t Test
d a = mean difference (series1)
d b = mean difference (series2)
Sda = Standard Deviation (series1)
Sdb = Standard Deviation (series 2)
na =number of samples analysed (series1)
nb =number of samples analysed (series2)
The difference in control group and cancer cases were considered significant if p < 0.01 15.
The carcinoma was detected under the guidance of Oncologist by necessary clinical laboratory investigation 16.
Results and Discussion
Tridax procumbans L, a wild medicinal plant was found to contain galactose specific lectin in leaves, stem and calyx. The lectins were purified to homogeneity by affinity chromatography and were found to have molecular weight of 23kD, 20kD, and 23kD for leaves, stem and calyx respectively. TPL-L TPL-S and TPL-C were able to agglutinate erythrocytes of breast cancer patients with less dilution than the normal (Table 1). The results illustrated in Table 1 appear to be statistically significant 16. These observations suggest that the receptor sites present on the erythrocytes of cancerous patients and normal persons may be different in number. David et al., (1978), also reported variations in lectin agglutination between erythrocytes of normal persons and cancer patients 17. The alteration in the cell membrane depends on the molecular basis. The changes on the erythrocyte surface may be due to the glycoprotein receptor sites present on the erythrocytes of cancerous patients and normal persons. The galactose epitopes may be increased in the erythrocytes of the breast cancer cases as compared to the normal individuals 16. Hemagglutination studies were also carried out by Mitchell et al., (1985) using H. pomatia agglutinin and P. vulgaris leucoagglutinin with erythrocytes of breast and colon cancer and reported the difference between the binding pattern in metastizing human breast and colon cancer with HPA, and PHA – L 18. Hasija (1991) reported that the binding pattern of cancer cells differ with different lectins. He found that out of thirty-five plant lectins, sixteen plant lectins showed no difference in the titre value (first group), however a marked difference was observed in the titre value with nine plant lectins (second group), while six lectins required a higher dilutions for agglutinating the cancer cells (third group) and four lectins required a lower dilution for agglutinating the cancer cells (fourth group), as compared to the erythrocytes of normal persons 12. Likewise T. procumbans lectins required a lower dilution for agglutinating the erythrocytes of breast cancer cases than the erythrocytes of normal persons. The difference between HAU due to lectin with the erythrocytes of normal and cancer patients were measured and significant results were obtained (Table 1). The results demonstrated that T. procumbans lectins required for agglutinating the erythrocytes of breast cancer patients were significantly more (p =0.001 i.e. < 0.05 St t test for TPL – L and TPL – C) and (p =0.02 i.e. less < 0.05 St t test for TPL – S) in stage I, and II as compared with control. T. procumbans lectins required for agglutinating the erythrocytes of breast cancer patients were still significantly more (p =0.001 i.e. < 0.05 St t test for TPL – L, TPL – C, and TPL – S) in stage III and IV, as compared with control. 95% confidence limit for normal subjects was calculated by taking mean 1.96 standard error which was found to be 2560 units for TPL – L, 1280 units for TPL – S and 2560 units for TPL – C. More than 85.68% values showed their clustering towards the below upper bound limit of the normal cases. However the HAU values of the cancer patient’s stage II and I were above the upper bound limit of normality range of 95% confidence limit as shown in Table 2. The Correlation Coefficient measured (Table 1) demonstrates that as the cancer stage increases the agglutination average titre decreases 16. Durgawale et al., (2001), also reported similar results with Synadenium root (Hook F) lectin and stated that more recept







