Apoorva Jain1 , Asha Neravi2
, Asha Neravi2 , Sunil Kumar Katti Sathyasheelappa2
, Sunil Kumar Katti Sathyasheelappa2 and Ajay Kumar Oli1*
and Ajay Kumar Oli1*
1Department of Biomedical Science, SDM Research Institute for Biomedical Sciences, Shri Dharmasthala Manjunatheshwara University, Sattur, Dharwad, Karnataka, India.
2Department of Obstetrics and Gynaecology, SDM College of Medical Sciences and Hospital, Shri Dharmasthala Manjunatheshwara University, Sattur, Dharwad, Karnataka, India.
Corresponding Author Email: ajay.moli@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/3105
Abstract
Polycystic ovary syndrome (PCOS) is an endocrine condition affecting women of reproductive age. The prevalence of PCOS is about 4-20% of women globally, leading to reproductive, hormonal, and metabolic abnormalities. PCOS result in Insulin resistance, raises the chronic metabolic risk such as hypertension, dyslipidaemia, cardiovascular disorders, type 2 diabetes and endometrial and/or breast cancer. The exact pathophysiology of PCOS is unknown, but it is evident from the study that lifestyle modifications and tailored diet therapy are the important therapeutic strategies in women. The diet treatment must be able to improve metabolic, reproductive, and insulin resistance functions, which can be reached through the design of a diet with low-calorie for loss of weight or maintain a balanced body mass index, limiting the intake of simple sugars and high-caloric food, restricting the intake of food with a low glycaemic index, reducing the consumption of saturated and trans fatty acids, consuming prebiotic food, and focusing on potential deficiency of vitamin D and omega-3 fatty acid. The purpose of this study is to present a critical literature analysis on dietary management as a novel therapy strategy for the PCOS.
Keywords
Glycaemic index; Insulin resistance; Nutritional management; Polycystic ovary syndrome; Pathophysiology; Vitamin D
Download this article as:| Copy the following to cite this article: Jain A, Neravi A, Sathyasheelappa S. K. K, Oli A. K. Nutritional Management of Polycystic Ovary Syndrome (PCOS)- A Review. Biomed Pharmacol J 2025;18(1). |
| Copy the following to cite this URL: Jain A, Neravi A, Sathyasheelappa S. K. K, Oli A. K. Nutritional Management of Polycystic Ovary Syndrome (PCOS)- A Review. Biomed Pharmacol J 2025;18(1). Available from: https://bit.ly/3ELihxY |
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrinological condition in women of fertile age, with the incidence rate of 16-40% depending on diagnostic criteria and the demographic studies.1
PCOS is a condition that causes female ovaries to enlarge and develop a significant number of cysts rather than a disease (more than 10). To develop a comprehensive and descriptive description of the condition, ovary wall thickening, a symptom of the disorder, occurs and stops the release of the mature follicles, or anovulation.2 About 70% of PCOS women are undiagnosed at early stage.3
According to the World Health Organization (WHO), 2012 survey 4–12% of women are affected with PCOS globally, and its ratio increased abruptly to 26% by 2020.4 The most contentious and difficult aspects of PCOS are its prevalence, diagnosis, aetiology, treatment, clinical procedures, psychological problems, and prevention.4,5
The National Institute of Health (NIH) in 1990, published criteria that provided a comprehensive and detailed explanation for PCOS diagnosis. Another diagnostic criterion, the Rotterdam Criteria 2003, the two of the three requirements listed below to be satisfied: 1) anovulation or oligomenorrhea; 2) hyperandrogenism, either clinically or biochemically; and 3) polycystic ovaries, which have 12 or more follicles per ovary, measuring 2 to 9 millimetres on ultrasonography. In 2006, the diagnostic guidelines were modified by the Androgen Excess Association (AES). The Rotterdam criteria of 2003 for PCOS, which are predicated on the coexistence of oligo-anovulation or polycystic ovaries and clinical or biochemical hyperandrogenism, were approved in 2012 by the National Institutes of Health.6,7
As per the retrospective cohort study conducted in San Francisco (USA), about 22.4% of PCOS females had androgenic alopecia (female baldness).8 Approximately 15-25% of females with PCOS show another clinical sign of hyperandrogenism, i.e., acne. Menstrual disorders range from amenorrhea (complete lack of period) to oligomenorrhea (delay in menstrual cycle of more than 35 days) to menorrhagia (heavy bleeding). 91% of women with irregular menstruation cycles are likely to have PCOS.9 Psychological problems like anxiety, depression, disturbed body image, and low self-esteem are also linked to PCOS. Nutritional management plays a critical role in managing PCOS. This review summarizes evidence-based dietary strategies to improve the clinical, metabolic, and reproductive outcomes in PCOS.
Causes and Risk Factors
Although the exact cause of PCOS is unknown, it results from a combination of causes, including genetic as well as environmental factors (Amenorrhea, dysmenorrhea, and anovulation) Due to insulin resistance and hyperinsulinemia, the function of the ovaries is disturbed by rising androgen levels, which lead to anovulation, suggesting that genetic factors are responsible for PCOS.10
Symptoms of menstrual disorders arise due to fluctuations in menstrual hormones like luteinizing hormone (LH), follicular stimulating hormone (FSH), oestrogen, testosterone, and factors affecting it.11 Predisposing risk factors for PCOS development include genetics, neuroendocrine variables, lifestyle/environment, and obesity. An unhealthy lifestyle, diet, or any infectious mediators and gut microbiome dysbiosis increase the risk of PCOS.
PCOS effects the concentrations of luteinizing hormone (LH), follicle-stimulating hormone (FSH), prolactin and gonadotropin-releasing hormone (GnRH).12 Environmental factors can result in genetic variation, disturbance of the metabolic and reproductive pathways, the development of PCOS phenotypes, and their associated complications.13
Allopathic Treatment of PCOS
The most common treatments for women with PCOS include medication, like clomiphene citrate, low-dose human menopausal gonadotropin or follicular stimulating hormone (FSH), aromatase inhibitors, laparoscopic ovarian drilling, and in vitro fertilization (IVF). Lifestyle changes, such as diet, exercise, and behavioural therapy, are also commonly used therapies.14
Pathophysiology of PCOS
PCOS could be caused by many factors as follows:
Hormonal imbalance
Insulin Resistance (IR)
Hyperandrogenism (HA)
The excess androgens during the early gonadotropin phase promote the development of antral and primordial follicles. Gonadotropin-releasing hormone (GnRH) secretion from the brain causes the pituitary gland to release gonadotropin hormone. Luteinizing hormone (LH) stimulates the luteinizing hormone (LH) receptor to increase androgen production in ovarian theca cells. In addition to acting on the follicular stimulating hormone (FSH) receptor in ovarian granulosa cells, follicular stimulating hormone (FSH) transforms androgens to estrogens, which encourage the growth of follicles.15 An excess of gonadotropin is believed to be the result of neuroendocrine system dysregulation, which throws the hypothalamic-pituitary-ovarian axis out of balance. Because the elevation in gonadotropin hormone encourages the synthesis of luteinizing hormone (LH) rather than follicular stimulating hormone (FSH), the luteinizing hormone to follicular hormone (LH: FSH) ratio is noticeably raised in PCOS.16
Because hyperinsulinemia directly imitates the action of LH and tangentially increases GnRH, it is the primary source of excessive androgen production.17,18 Insulin lowers sex hormone-binding globulin (SHBG), a crucial blood protein that controls testosterone levels. Because free androgens are responsible for clinical symptoms like hirsutism, alopecia, and acne, a lower sex hormone-binding globulin (SHBG) would result in a greater concentration of these hormones.19 Patients with PCOS have a higher chance of diabetes and cardiovascular disease because of insulin resistance, which can also result in dyslipidaemia 20. Several trials showed that reducing insulin resistance over time would reduce the excess androgens and improve the PCOS condition.15
The elevated androgen secretion resulting from ovarian dysfunction leads to abnormal follicular development and ovulatory dysfunction, resulting in polycystic ovary disease.6 Anti-Mullerian hormone (AMH), produced by immature antral follicles and accumulating in the ovaries of PCOS patients, exacerbates ovarian dysfunction by altering the follicular microenvironment and/or causing gonadotropin hormone (GnRH) hypersecretion. Dysregulation of gonadotropin hormone secretion driven by hyperandrogenism, particularly the excessive secretion of luteinizing hormone (LH), can be at least partially attributed to aberrant negative or positive feedback by progesterone and estrogen.13 The dysregulation of follicular development is further exacerbated by elevated levels of LH, leading to an imbalance in the luteinizing hormone to follicular stimulating hormone (LH/FSH) ratio and the hypersecretion of androgens from thecal cells.21
Nutritional Management of Polycystic Ovary Syndrome
Diet and lifestyle choices significantly impact androgen activity, especially concerning body weight, insulin resistance, inflammation, and oxidative stress. Between 30-75% of women affected by PCOS are obese; even when they are not obese, these women frequently have excess body fat, particularly central adiposity.22
Low Glycaemic Food
The Glycaemic Index (GI) measures how rapidly the carbohydrate and sugar content of food is metabolized and absorbed into the blood. High glycaemic index foods cause blood sugar levels to rise quickly and drastically, whereas low glycaemic index foods result in gradual, smaller increases.23 Low glycemic diet are vital for reducing risks and improving clinical and biochemical outcomes in PCOS. These diets, characterized by minimally processed, fiber-rich foods like whole wheat bread, sweet potatoes, chickpeas, lentils, apples and oats, help manage appetite and carbohydrate cravings while reducing dyslipidaemia and weight gain. Sustainable evidence supports their benefits for managing the PCOS effectively.24 Research has shown that low-glycemic index diets have many benefits. This diet can reduce insulin resistance, however items with a high glycemic index have the opposite effect.25 According to epidemiological study, a diet with low glycaemic index has been associated with a lower risk of cardiovascular disorder, type 2 diabetes, insulin resistance and incidence of endometrial, breast, and ovarian cancers.26 Therefore, maintaining metabolic health is more dependent on the type of carbohydrate consumed than on the amount consumed.
Table 1: Sources of Low, Moderate and High glycemic index foods
|
Sources |
Index- Low Glycemic |
Index- Moderate Glycemic |
Index-High Glycemic |
References |
|
Vegetables |
Asparagus, Broccoli, cucumber, Cabbage, Eggplant, Green beans Lettuce, Mushrooms, Onions Raw carrots, Tomatoes. |
Beetroot |
Potato, Pumpkin |
[27], [28] |
|
Fruits |
Apples, Dried Apricots, Cherries, Grapes, Guava, Kiwi, Oranges, Plums, Peaches, Pomegranate, Strawberries |
Bananas, Figs, Mango, papaya, Pineapple, Raisins |
Dates, Watermelon |
[27],[29] |
|
Dairy |
Buttermilk, Curd, Skim Milk, Soy Milk, Whole milk, Paneer, Yogurt |
Ice-cream |
– |
[27],[28] |
|
Beans/Legumes |
Chickpea, Red & Green Lentils, Kidney beans |
– |
– |
[27],[28], |
|
Starch |
Barley, Quinoa, Popcorn, Peas, Sweet corn, Rice bran |
Basamati rice, Brown rice, Instant Cooking Oats, Sweet potato |
Carrots, White rice, White bread, Potato |
[28],[29] |
Vitamin D
Vitamin D deficiency has been reported in about 67-85% of individuals with PCOS.29 There is evidence to suggest that obesity and low vitamin D levels are related; inversely, a vitamin D deficiency may indicate an excess of adipose tissue. The higher serum 25(OH)D concentrations is associated with improved markers of ovarian reserve, including higher levels of anti-Mullerian hormone (AMH), suggesting that sufficient vitamin D levels could support ovarian function and potentially protect against premature ovarian depletion,30,31 Vitamin D supplementation is beneficial in reducing insulin. Research has shown that vitamin D is involved in several metabolic processes, including the metabolism of insulin, and that a lack of vitamin D influencing the etiology of insulin resistance and PCOS.32, 33According to a recent review paper by Thomson et al., vitamin D levels and hormonal and metabolic issues in PCOS patients may be related. Although the exact mechanism causing this impact is yet unknown, it has been suggested that ovarian malfunction may play a part in the processes that control apoptosis.34 Furthermore, because of its immunomodulatory function, vitamin D deficiency can trigger inflammatory reactions that result in insulin resistance.35 Low vitamin D levels have been linked to PCOS in a number of studies. Consequently, vitamin D supplementation might be useful in changing these patients’ hormones and metabolism.36
Additionally, vitamin D deficiency is correlated with elevated androgen levels, including testosterone and dehydroepiandrosteronesulfate (DHEA-S), which contribute to hyperandrogenism and associated symptoms such as hirsutism. This deficiency is also connected to other PCOS complications, including impaired follicle maturation and increased risk of developing metabolic syndrome and cardiovascular diseases.
Calcium
The observed decrease in circulating androgens may provide evidence that calcium and vitamin D directly impact the pathway leading to the production of ovarian and/or adrenal steroid hormones. Research indicates that 1,25-Dihydroxyvitamin D enhances insulin secretion by increasing intracellular calcium levels in pancreatic β-cells. Vitamin D enhances the expression of calcium-binding proteins in β-cells, which is crucial for insulin secretion. A positive association between serum calcium levels and insulin sensitivity was found in a healthy population. This study highlighted that optimal calcium levels might play a role in maintaining insulin sensitivity and glucose metabolism.37 Additionally, it has been noted that the level of glucose and phosphorus in PCOS women with high Body mass Index (BMI) correlated positively with vitamin D levels and negatively with insulin and IR. The production of androgens and oocyte maturation is influenced by calcium and vitamin D metabolism, according to the data. Malondialdehyde (MDA) concentrations, free testosterone, plasma total antioxidant capacity (TAC), and serum dehydroepiandrosteronesulfate (DHEAS) levels all improved when combination supplementation of vitamin D-K-calcium was used for treatment for eight weeks to vitamin D-deficient women with PCOS.38
Prebiotics
Prebiotics are non-living, indigestible fibers that can promote the development and action of advantageous microorganisms in the gut.39 Women with PCOS have a less different gut microbiota than women without the condition, and there is evidence that their intestinal permeability is higher.40 Hyperandrogenism and elevated systemic inflammation have been associated with this decrease in gut bacteria diversity. The dysbiosis of the gut microbiota has an impact on the pathogenesis of PCOS. One of the processes is dysbiosis in the gut microbiota, which activates the host’s immune system.
The insulin receptor function is disrupted by the activation of immune system which results in hyperinsulinemia. This, in turn, impacts the ovarian androgen production and impedes the proper development of follicles. A novel strategy to treating PCOS is the use of medicines that target the microbiome. Thus, probiotics, prebiotics, and symbiotic represent novel PCOS therapeutic alternatives.41
Fatty Acids
Consuming an appropriate quantity of dietary polyunsaturated fatty acids (PUFAs) benefits individuals with PCOS who have conditions such as insulin resistance, lowered vascular endothelial function, and dyslipidaemia. One of the polyunsaturated fatty acids (PUFAs), Omega-3 fatty acids, is less abundant and found only in deep-sea fish and plant sources (e.g., green leafy vegetables, seeds, and vegetable oils).42 Recent research from the Scientific Congress of the American Society of Reproductive Medicine revealed omega-3s are advantageous for enhancing fertility and chances of conception in women with PCOS.43 In the research by Oner and Muderris, insulin levels considerably dropped after consuming omega-3 fatty acids for six months, but there were no significant changes in glucose levels or the Homeostatic Model Assessment for Insulin Resistance (HOMA) index.44 Levels of omega-3 are linked to the decreased availability of arachidonic acid. Numerous mechanisms by which insulin resistance affects the hormonal system have been determined. Consuming unsaturated fats foods lowers the risk of chronic diseases, but diets with saturated and trans-fatty acids, impair insulin sensitivity and raise the risk of type 2 diabetes, metabolic syndrome, and cardiovascular disease.45 As shown in figure 1, this is particularly true for diets that contain fatty acids and omega-3 unsaturated fatty acids, which can reduce many risk of metabolic disorders like high serum lipid levels, insulin resistance, and impaired endothelial function42 that are seen in women with PCOS.
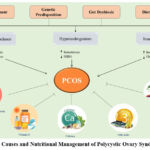 |
Figure 1: The Causes and Nutritional Management of Polycystic Ovary Syndrome (PCOS)
|
Conclusion
PCOS is quite common among women of reproductive age around the globe. Additionally, its effects are substantial enough to support a review of potential dietary interventions. PCOS is most frequently linked with obesity, but there are also patients with normal weight who have the condition. The goal of intervention in cases of overweight or obesity is to lower body weight and keep it within the usual range. It has been suggested that dietary minerals directly impact oxidative stress, inflammation, and metabolic control. Various dietary regimens have been recommended to manage PCOS. Nutritional management supports the control of the inflammatory state, insulin resistance, and hyperandrogenaemia. The importance of an approach that focuses on nutrition and exercise has become evident. Considering that drug therapy has only shown to be successful in the short term, a personalized diet and regular exercise are likely the only strategies that will have long-lasting effects.
Acknowledgment
We thank Shri Dharmasthala Manjunatheshwara University, Sattur, Dharwad, Karnataka-India, for their constant support and encouragement.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The author(s) do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials
Author Contributions
AJ: Collection of the literature and preparation of the initial manuscript. AN: Manuscript preparation and revision of the manuscript. SKK: Revision of the manuscript. AKO: Study design, data collection, and script finalization. All authors read and approved the final version of the manuscript. Apoorva Jain and Ajay Kumar Oli are both guarantors of the paper.
References
- Nagarathna P., Rajan P.R. and Koneri R. A. Detailed study on Polycystic Ovarian Syndrome and its treatment with Natural Products. International journal of toxicological and pharmacological research. 2014;5(4):109-120.
- Teede H.J., Misso M.L., Costello M.F., Dokras A., Laven J., Moran L., Piltonen T. and Norman R. J. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertility and Sterility. 2018;110(3): 364-379. doi: 10.1016/j.fertnstert.2018.05.004.
CrossRef - Deswal R., Narwal V., Dang A. and Pundir C.S. The prevalence of polycystic ovary syndrome: A brief systematic review. Journal of Human Reproductive Sciences. 2020;13(4): 261-271.doi: 10.4103/jhrs.JHRS_95_18.
CrossRef - Zeng L.H., Rana S., Hussain L., Asif M., Mehmood M.H., Imran I., Younas A., Mahdy A., Al-Joufi F.A. and Abed SN. Polycystic Ovary Syndrome: A Disorder of reproductive age, Its pathogenesis, and a discussion on the emerging role of herbal remedies. Frontiers in Pharmacology. 2022; 13:874914. doi: 10.3389/fphar.2022.874914.
CrossRef - Maria T. and Catherine M. G. Diagnosis and Management of Polycystic Ovary Syndrome in Adolescents. Pediatrics. 2020; 145 (2): 210–218.
CrossRef - Azziz R. Polycystic Ovary syndrome. Obstetrics & Gynecology. 2018;132(2): 321-336.doi: 10.1097/AOG.0000000000002698.
CrossRef - Azziz R., Carmina E., Chen Z., Dunaif A., Laven J.S., Legro R.S., Lizneva D., Natterson H. B., Teede H.J. and Yildiz B.O. Polycystic ovary syndrome. Nature Reviews Disease Primers. 2016; 2: 157-160. doi:10.1038/nrdp.2016.57
CrossRef - Taieb A. and Feryel A. Deciphering the role of androgen in the dermatologic manifestations of Polycystic Ovary Syndrome patients: A state-of-the-art Review. Diagnostics. 2024; 14(22): 2578.
CrossRef - Bulsara J., Patel P., Soni A. and Acharya S. A review: Brief insight into polycystic ovarian syndrome. Endocrinology and Metabolism. 2021;3:1-7.doi: 10.1016/j.endmts.2021.100085
CrossRef - Ajmal N., Zeib Khan S. and Shaikh R. Polycystic ovary syndrome (PCOS) and genetic predisposition: A review article. European Journal of Obstetrics &Gynecology and Reproductive Biology: X. 2019; 3:1-6. doi:10.1016/j.eurox.2019.100060
CrossRef - Reed B.G. and Carr BR. The Normal Menstrual Cycle and the Control of Ovulation. In: Feingold KR, Anawalt B, Blackman MR, et al., Endotext. South Dartmouth (MA): MDText .com, Inc.; 2000. https://www.ncbi.nlm. nih.gov/books/NBK279054/
- Myers S.H., Montanino O.M., Nordio M. and Unfer V. PCOS phenotype focus: phenotype D under the magnifying glass. Archives of Gynecology and Obstetrics. 2024;309(6):2307-2313. doi: 10.1007/s00404-024-07408-2.
CrossRef - Dewailly D., Barbotin A.L., Dumont A., Catteau J. S. and Robin G. Role of Anti-Mullerian hormone in the pathogenesis of Polycystic Ovary Syndrome. Frontiers in Endocrinology. 2020; 11:641. doi: 10.3389/fendo.2020.00641
CrossRef - Badawy A. and Elnashar A. Treatment options for polycystic ovary syndrome. International Journal of Women’s Health. 2011; 3:25-35. doi: 10.2147/IJWH.S11304.
CrossRef - Orlowski M. and Sarao M.S. Physiology, Follicle Stimulating Hormone. StatPearls. Treasure Island (FL): StatPearls Publishing; 2024. https://www.ncbi.nlm.nih.gov/books/NBK535442/
- Ashraf S., Nabi M., Rasool S.A., Rashid F. and Amin S. Hyperandrogenism in polycystic ovarian syndrome and role of CYP gene variants: a review. Egyptian Journal of Medical Human Genetics. 2019; 20(1): 25-30.doi:10.1186/s43042-019-0031-4.
CrossRef - Walters K.A., Gilchrist R.B., Ledger W.L., Teede H.J., Handelsman D.J. and Campbell R.E. New perspectives on the pathogenesis of PCOS: neuroendocrine origins. Trends in Endocrinology & Metabolism. 2018;29(12): 841-852. doi: 10.1016/j.tem.2018.08.005.
CrossRef - Puttabyatappa M. and Padmanabhan V. Ovarian and extra-ovarian mediators in the development of polycystic ovary syndrome. Journal of Molecular Endocrinology. 2018;61(4):161-184. doi: 10.1530/JME-18-0079.
CrossRef - Qu X. and Donnelly R. Sex Hormone-Binding Globulin (SHBG) as an rarly Biomarker and therapeutic target in Polycystic Ovary Syndrome. International Journal of Molecular Sciences. 2020;21(21):8191. doi: 10.3390/ijms21218191.
CrossRef - Wang E.T., Calderon M. R., Cedars M.I., Daviglus M.L., Merkin S.S., Schreiner P.J., Sternfeld B., Wellons M., Schwartz S.M., Lewis C.E., Williams O.D., Siscovick D.S. and Bibbins-Domingo K. Polycystic ovary syndrome and risk for long-term diabetes and dyslipidemia. Obstetrics & Gynecology. 2011;117(1):6-13. doi: 10.1097/AOG.0b013e31820209bb.
CrossRef - Rocha A.L., Oliveira F.R., Azevedo R.C., Silva V.A., Peres T.M., Candido A.L. and Gomes KB, Reis FM. Recent advances in the understanding and management of polycystic ovary syndrome. F1000Research. 2019; 8:1-11. doi:10.12688/f1000research.15318.1.
CrossRef - Szczuko M., Kikut J., Szczuko U., Szydłowska I., Nawrocka R. J., Ziętek M., Verbanac D., Saso L. Nutrition Strategy and life style in Polycystic Ovary Syndrome-Narrative Review. Nutrients. 2021;13(7):2452. doi: 10.3390/nu13072452.
CrossRef - Nutrition Guide for clinician. Polycystic Ovary Syndrome. https://nutritionguide.pcrm.org/nutritionguide/ view/Nutrition_Guide_for_Clinicians/1342095/all/Polycystic_Ovary_Syndrome.,2023.
- Berkshire Healthcare Foundation Trust Dietitians. Polycystic Ovary Syndrome (PCOS) dietary advice. https://www.royalberkshire.nhs.uk/media/afta2biw/pcos-dietary-advice_nov22.pdf..2022.
- Saadati N., Haidari F., Barati M., Nikbakht R., Mirmomeni G., and Rahim F. The effect of low glycemic index diet on the reproductive and clinical profile in women with polycystic ovarian syndrome: A systematic review and meta-analysis. Heliyon. 2021;7(11): 2405-8440. doi: 10.1016/j.heliyon. 2021.e08338.
CrossRef - Mohd A.Y., Bushra. and Tanzim. Role of diet in the management of polycystic ovarian syndrome (PCOS). International Journal of Advanced Research. 2019;7(4):674-680. doi:10.21474/IJAR01/8870.
CrossRef - Magic kitchen. Low glycemic index foods help control blood sugar. https://www.magickitchen.com/info/lgi-foods-help-control-blood-sugar.html. ,2023.
- Clar C., Al-Khudairy L., Loveman E., Kelly S.A., Hartley L., Flowers N., Germanò R., Frost G. and Rees K. Low glycaemic index diets for the prevention of cardiovascular disease. Cochrane Database of Systematic Reviews. 2017;7(7):CD004467. doi: 10.1002/14651858.CD004467.pub3.
CrossRef - Ajith and Meera. Nutritional Management of PCOS: A review article. Journal of Personality and Social Psychology. 2022;6(8):8979-8984.
- Trummer C., Pilz S., Schwetz V., Obermayer P. B. and Lerchbaum E. Vitamin D, PCOS, and androgens in men: a systematic review. Endocrine Connections. 2018;7(3): 95-113.
CrossRef - Watson R.R. and Zibadi S. Handbook of Nutrition in Heart Health. Human Health Handbooks. 2023;4:853-859.
- Lagowska K., Bajerska J. and Jamka M. The Role of Vitamin D oral supplementation in insulin resistance in women with Polycystic Ovary Syndrome: A Systematic Review and Meta-analysis of randomized controlled trials. Nutrients. 2018 ;10(11):1637. doi: 10.3390/nu10111637.
CrossRef - Luisi S., Luddi A., Piomboni P., Governini L. And De Leo V. PCOS Physiopathology and Vitamin D Deficiency: Biological Insights and Perspectives for Treatment. Journal of Clinical Medicine. 2022;11(15):4509.
CrossRef - He C., Lin Z., Robb S.W. and Ezeamama A.E. Serum Vitamin D Levels and Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Nutrients. 2023;15(3):576.
- Sung C.C., Liao M.T., Lu K.C. and Wu C.C. Role of vitamin D in insulin resistance. Journal of Biomedicine and Biotechnology. 2012; 2012: 634195. doi: 10.1155/2012/634195.
CrossRef - Mohan A., Haider R., Fakhor H., Hina F., Kumar V., Jawed A., Majumder K., Ayaz A., Lal PM., Tejwaney U., Ram N. and Kazeem S. Vitamin D and polycystic ovary syndrome (PCOS): a review. Annals of Medicine and Surgery. 2023;85(7):3506-3511. doi: 10.1097/MS9.0000000000000879.
CrossRef - Wu X., Han T., Gao J., Zhang Y., Zhao S., Sun R., Sun C., Niu Y. and Li Y. Association of serum calcium and insulin resistance with hypertension risk: A Prospective population-Based Study. Journal of the American Heart Association. 2019;8(1): e009585. doi: 10.1161/JAHA.118.009585.
CrossRef - Maestro B., Molero S., Bajo S., Dávila N. and Calle C. Transcriptional activation of the human insulin receptor gene by 1,25-dihydroxyvitamin D3. Cell Biochemistry and Function.2019;37(2): 127-132. doi: 10.1002/cbf.3386
CrossRef - Khan A.H., Khan F.A., Mehmood T. and Sultan A. Serum calcium levels and their correlation with insulin resistance and obesity in the adult population. Journal of Clinical Endocrinology & Metabolism. 2020; 105(3): 896-904. doi: 10.1210/clinem/dgz181.
CrossRef - Shojaeian Z., Sadeghi R. and Roudsari R. Calcium and vitamin D supplementation effects on metabolic factors, menstrual cycles and follicular responses in women with polycystic ovary syndrome: A systematic review and meta-analysis. Caspian Journal of Internal Medicine. 2019;10(4):359-369. doi:10.22088/cjim.10.4.359.
- Yurtdas G. and Akdevelioglu. A New approach to Polycystic Ovary Syndrome: The Gut Microbiota. Journal of the American College of Nutrition. 2020.39(4):371-382. org/10.1080/07315724.2019.1657515
CrossRef - Lu L., Li X., Lv L., Xu Y., Wu B. and Huang C. Associations between omega-3 fatty acids and insulin resistance and body composition in women with polycystic ovary syndrome. Frontiers in Nutrition. 2022; 9:1016943. doi:10.3389/fnut.2022.1016943.
CrossRef - Trop-Steinberg S., Gal M., Azar Y., Kilav-Levin R. And Heifetz EM. Effect of omega-3 supplements or diets on fertility in women: A meta-analysis. Heliyon. 2024; 10(8): e29324. doi: 10.1016/j.heliyon. 2024.e29324.
CrossRef - Yadav M.K., Kumari I., Singh B., Sharma K.K. and Tiwari S.K. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Applied Microbiology and Biotechnology. 2022; 106; 505–521. https://doi.org/10.1007/s00253-021-11646-8
CrossRef - Salek M., Clark C., Taghizadeh M. and Jafarnejad S. N-3 fatty acids as preventive and therapeutic agents in attenuating PCOS complications. EXCLI Journal.2019; 18:558-575. doi:10.17179/excli2019-1534.
Abbreviations
PCOS- Polycystic Ovary Syndrome
WHO- World Health Organization
NIH- National Institute of Health
AES- Androgen Excess Association
LH- luteinizing hormone
FSH- Follicular Stimulating Hormone
GnRH- gonadotropin-releasing hormone
IVF- In-vitro fertilization
IR- Insulin Resistance
HA-Hyperandrogenism
SHBG- sex hormone-binding globulin
AMH-Anti-Mullerian hormone
GI-Glycaemic index
HOMA-IR-Homeostatic model assessment for insulin resistance
MDA- malondialdehyde
TAC- Total antioxidant capacity
DHEAS- dehydroepiandrosteronesulfate
PUFA- polyunsaturated fatty acid








