Bindu Dhanapal1* , Mymoonah Risha1
, Mymoonah Risha1 , Vyshnavee Subramaniyan2
, Vyshnavee Subramaniyan2 and Ezhilnilavan Murugesan2
and Ezhilnilavan Murugesan2
1Department of Microbiology, Sree Balaji Medical College and Hospital, Chrompet, Chennai, BIHER, Tamilnadu, India.
2Government Medical College, Tiruchirapalli, Tamilnadu, India
Corresponding Author E-mail: mail2bindhu@rediffmail.com
DOI : https://dx.doi.org/10.13005/bpj/3129
Abstract
Introduction: Mucormycosis is a rare but deadly fungal infection that affects immunocompromised patients, particularly those with uncontrolled diabetes, excessive corticosteroid use, and COVID-19. Aim and objective: This study aimed to evaluate the sensitivity of modified potassium hydroxide-lactophenol cotton blue (KOH-LPCB) staining for the identification of fungal species using modified KOH-LPCB staining. Materials and methods: One hundred fifty-three tissue samples from clinically suspected mucormycosis cases were analyzed using KOH and KOH-LCPB mounts. Results: Rhizopus spp. was the most common fungal isolate, accounting for 48 of the 83 positive cases (57.8%). The modified KOH-LCB method demonstrated a sensitivity of 92% for detecting fungal elements, making it a valuable tool for early diagnosis in resource-limited settings. Conclusion: This study underscores the significant association between mucormycosis and COVID-19, particularly in patients with uncontrolled diabetes and recent corticosteroid use. In addition, two rare species, Cokeromyces recurvatus and Syncephalastrum racemosum, were isolated. The presence of these rare species emphasizes the need for comprehensive fungal diagnostics to avoid misdiagnoses.
Keywords
Diabetes mellitus; Covid-19; KOH-Calcofluour; KOH-LPCB; Mucormycosis
Download this article as:| Copy the following to cite this article: Dhanapal B, Risha M, Subramaniyan V, Murugesan E. Modified Potassium Hydroxide Method for Early Diagnosis of Mucormycosis. Biomed Pharmacol J 2025;18(1). |
| Copy the following to cite this URL: Dhanapal B, Risha M, Subramaniyan V, Murugesan E. Modified Potassium Hydroxide Method for Early Diagnosis of Mucormycosis. Biomed Pharmacol J 2025;18(1). Available from: https://bit.ly/42GrfXm |
Introduction
Mucormycosis is an uncommon but fatal angioinvasive fungal disease caused by the fungal mold of zygomycetes, order Mucorales1 which includes 12 genera: Mucor, Rhizopus, Rhizomucor, Adsidia, and Cunninghamella. Actinomucor, Apophysomyces, Cokeromyces, Lichtheimia, Mycotypha, Saksenaea, Syncephalastrum, and Thamnostylum. These fungi are ubiquitous in the environment, particularly in soils and in decaying organic matter. Approximately 47,500 fungal infections have been reported in India.2 Mucormycosis primarily affects immunocompromised individuals, including those with uncontrolled diabetes, malignancies, or those undergoing immunosuppressive therapy. The clinical presentation of mucormycosis can vary, but it often involves the rhino-orbital-cerebral, pulmonary, gastrointestinal, or cutaneous systems. Early diagnosis and prompt antifungal treatment, typically with amphotericin B, are crucial to improve patient outcomes. The COVID-19 pandemic has led to a significant increase in mucormycosis cases, especially in India.3 India has approximately 70 times more COVID-19-associated mucormycosis (CAM) than the rest of the world.4 Several factors contribute to this increase, including the use of corticosteroids to manage severe COVID-19, prolonged hospitalization, and the presence of comorbidities such as diabetes mellitus. India, which has one of the highest global burdens of diabetes, has reported an alarming surge in mucormycosis cases among COVID-19 patients. The first case of COVID-19-associated mucormycosis was reported in Chile.5 India has been the leading cause of mucormycosis during the second wave of COVID-19 due to delta strain.6
Mucormycosis is a severe opportunistic infection with high fatality and mortality rates that require prompt recognition and urgent intervention.7 The three major risk factors for mucormycosis development are uncontrolled diabetes mellitus, steroid use, and immune system.8 The outcomes associated with poor glycemic control are worse even with aggressive medical and surgical interventions. A study conducted in India during the second wave of COVID-19 revealed that most mucormycosis cases occurred in males, with diabetes being the predominant underlying condition. The clinical presentation of mucormycosis in COVID-19 patients typically includes rhino-orbital-cerebral involvement, characterized by symptoms such as nasal congestion, facial swelling, headache, and visual disturbances. Prompt diagnosis and aggressive management, including surgical debridement and antifungal therapy, are crucial for improving the outcomes in these patients.
Cokeromyces recurvans (C .recurvatus) is a lesser-known zygomycete that can cause mucormycosis, although reports are rare. This dimorphic fungus is known to cause infections predominantly in immunocompromised individuals. It has been isolated from lizards, soil, and rodent excrement, as well as from humans. There were only 14 instances of C .recurvatus documented to date, with eight (57%) involving the female vaginal tract9,10. In this instance, the majority of gynecologic cases were unintentionally discovered in asymptomatic patients. The other samples were linked to C .recurvatus includes lung, pleural, peritoneal, urine, and stool fluids, as well as the fluid from an intra-abdominal abscess. Both immunocompromised and immunocompetent individual were affected by C .recurvatus, and its presence can have a variety of pathological consequences, starting with asymptomatic instances.
Syncephalastrum racemosum (S. racemosum) is a rare species of fungus belonging to the order Mucorales. It is characterized by broad, septate hyphae with branched sporangiophores and mero sporangia containing chains of sporangiospores. Although not commonly pathogenic, S. racemosum has been documented in various infections, particularly in immunocompromised individuals.11,12 Its identification alongside other common mucormycosis-causing fungi underscores the diversity of fungal pathogens that can complicate COVID-19 infections.
Although known fungal identification methods are sensitive and specific, early diagnosis of mucormycosis is crucial for initiating treatment before culture results are available, especially in resource-limited settings. This study aimed to evaluate the sensitivity and identification of mucormycosis fungi using modified potassium hydroxide-lactophenol cotton blue (KOH-LPCB) staining. In addition, it seeks to identify rare fungal species and assess their impact on health.
Materials and Methods
Study Design
This Laboratory-based observational study was conducted in a tertiary care hospital to identify the fungal species causing mucormycosis in COVID-19 patients from May 2021 to December 2021. One hundred and fifty-three tissue samples received in the Microbiology laboratory from the patients presented with one or more of the following symptoms: headache, loss of vision, maxillary sinusitis, headache, facial cellulitis, necrosis of palatal bone/mucosa (clinically suspected mucormycosis cases) were analyzed.
Sample Collection
Tissue samples were obtained from patients diagnosed with COVID-19 who presented with symptoms indicative of mucormycosis. Samples included biopsy tissues from the nasal cavity, sinuses, orbital region, and brain collected from three departments: ENT, Ophthalmology, and Microbiology. Samples taken from known cases of previous COVID-19 infection or their reverse transcriptase polymerase chain reaction (RT-PCR) COVID-19 test upon admission revealed positive results. All the patients were treated with corticosteroids as part of the standard blanket Covid-19 drug regime.
KOH Mount
Tissue from patients was added to 10% KOH and incubated for 24 h in a sterile test tube. Then a drop of tissue material with 10% KOH from the tube was added to a glass slide covered with a coverslip and viewed under low power (10x) and high power (40x) under a microscope.
Modified KOH-LPCB mount
Tissue from patients was added to 10% KOH and incubated for 24 h in a sterile test tube. A drop of tissue material with10% KOH from the test tube was placed on a glass slide and a drop (50µl) of lactophenol cotton blue (LPCB) was added to it, covered with a coverslip and incubated for 10 min to increase the sensitivity of the test. The glass slide was then examined under low power (10x) and high power (40x) under a microscope. Colonies had a woolly or cottony texture, with distinct radial grooves.
Isolation and identification
Tissue samples were inoculated on Sabouraud dextrose agar. Organisms were identified based on the colony characteristics of each fungus.13
Fungal Examination
Stained samples were examined under a microscope for the presence of fungal hyphae. Morphological characteristics such as hyphal width, branching pattern, and spore formation were used to identify fungal species.13
Mucor spp.
KOH Mount: Broad, non-septate hyphae with irregular branching.
Hyphae appear ribbon-like and often folded.
Modified KOH (KOH-LPCB): Hyphae stained blue, providing clearer visualization. Sporangiophores with terminal sporangia containing sporangiospores were visible.
SDA Colony Description: Rapid growth, quickly filling the Petri dish in a few days. The colonies were initially white, becoming gray to brownish with age.
Fluffy or cottony texture with radial grooves.
Rhizopus spp.
KOH Mount: Broad, non-septate hyphae with right-angle branching. The presence of rhizoids (root-like structures) and sporangiophores.
Modified KOH (KOH-LPCB): Blue-stained hyphae with distinct sporangiophores. Spherical sporangia containing sporangiospores at the tips of sporangiophores
SDA Colony Description: Rapid growth, quickly filling Petri dish. The colonies were initially white, turning gray or yellowish-brown with age.
Dense, woolly, or cottony texture.
Rhizomucor spp.
KOH Mount: Broad, sparsely septate hyphae with irregular branching. Presence of sporangiophores with sporangia at tip.
Modified KOH (KOH-LPCB): Hyphae stained blue, sporangiophores and sporangia were more easily visualized.
Sporangia are often smaller compared to Rhizopus.
SDA Colony Description: Rapid growth with colonies filling the dish.
Colonies are white, turning grayish with age.
Fluffy or cottony texture, similar to Mucor and Rhizopus but often more compact.
Cokeromyces recurvatus
KOH Mount:
Broad, sparsely septate hyphae with unique recurved (curved back) sporangiophores.
Presence of sporangia at the tips of recurved sporangiophores.
Modified KOH (KOH-LPCB):
Blue-stained hyphae with distinctly recurved sporangiophores.
Sporangia and sporangiospores are more easily visualized.
SDA Colony Description:
Moderate growth rate.
Colonies are initially white, turning grey or brown with age.
Colonies have a velvety or woolly texture with distinct zones of growth.
Syncephalastrum racemosum
KOH Mount:
Broad, septate hyphae with branched sporangiophores.
Presence of mero sporangia containing chains of sporangiospores.
Modified KOH (KOH-LPCB):
Blue-stained hyphae with clearer mero sporangia.
Chains of sporangiospores are more distinctly visible.
SDA Colony Description:
Rapid growth with colonies filling the dish.
Colonies are white, turning gray to black with age.
Results and Discussion
Candida and Aspergillus were the common fungus associated with COVID-19. However the increase in the incidence of the “Black fungi” was alarming due to its invasiveness, morbidity, and mortality. It is most common in immunocompromised conditions where there is malfunctioning of macrophages like in the case of diabetes, transplant recipients, malignancies, etc.
The data presented in Table 1 showed the presence of Mucor 22(26.5 %), Rhizopus spp. 48 (57.8 %), Rhizomucor spp. 6 (7.2 %), Aspergillus fumigatus 5 (6 %), Cokeromyces recurvatus 1 (1.2 %), Syncephalastrum racemosum 1 (1.2 %) and the corresponding figures highlight several important findings regarding the incidence and identification of fungal species in COVID-19-associated mucormycosis cases.
Table 1: Distribution of fungus from samples
| S.No | Fungal isolate | Number of strains |
| 1 | Mucor spp. | 22 |
| 2 | Rhizopus spp. | 48 |
| 3 | Rhizomucor spp. | 6 |
| 3 | Aspergillus fumgiatus | 5 |
| 4 | Cokeromyces recurvatus | 1 |
| 5 | Syncephalastrum racemosum | 1 |
| Total | 83 |
Demographics: Eighty-three patients with suspected mucormycosis were included in the study. The majority were males (60%) with a mean age of 55 years similar to few study14. A significant number of patients had underlying conditions such as diabetes mellitus (75%), recent corticosteroid use (65%), and COVID-19 infection (100%).
Predominance of Rhizopus spp: Of the 153 samples analyzed, 83 (54.24%) yielded fungal growth, while 70 (45.75%) showed no growth. The distribution of fungal isolates is summarized in Table 1: Mucor spp.: 22 isolates (26.5%), Rhizopus spp.: 48 isolates (57.8%), Rhizomucor spp.: 6 isolates (7.2%), Aspergillus fumigatus: 5 isolates (6.0%), C .recurvatus: one isolate (1.2%), S .racemosum: one isolate (1.2%). The most common fungal isolate was Rhizopus spp., which accounted for 48 of 83 positive cases (57.8%). This aligns with the literature2,15,16,17,18 which has identified Rhizopus as a leading cause of mucormycosis in immunocompromised patients, particularly those with uncontrolled diabetes and those undergoing corticosteroid therapy.
Efficacy of KOH and Modified KOH-LPCB Mounts: Figure 1 illustrates the use of KOH and modified KOH-LPCB mounts for detecting broad aseptate hyphae, which are characteristic of mucormycosis. The modified KOH-LPCB method demonstrated similar sensitivity to calcofluor staining, making it a valuable tool for early diagnosis in resource-limited settings. The KOH-LPCB method demonstrated a sensitivity of 92% for detecting fungal elements. The KOH-Calcofluor White stain had a sensitivity of 95%. Both methods were effective; however, the KOH-LPCB method was preferred because of its simplicity and cost-effectiveness.
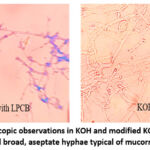 |
Figure 1: Microscopic observations in KOH and modified KOH-LPCB mounts showed broad, aseptate hyphae typical of mucormycosisClick here to view Figure |
Identification of rare species: This study identified two rare fungal species, C .recurvatus (figure 2) and S .racemosum (figure 2), each isolated in one case. The presence of these rare species emphasizes the diversity of fungal pathogens that can complicate COVID-1911, suggesting the need for comprehensive diagnostic approaches to avoid misdiagnosis.
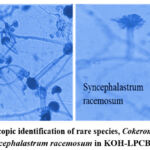 |
Figure 2: Microscopic identification of rare species, Cokeromyces recurvatus, and Syncephalastrum racemosum in KOH-LPCB mount.Click here to view Figure |
Clinical correlation with COVID-19: The discussion notes a higher incidence of mucormycosis in male patients (68.7%) than in female patients (31.3%), which is consistent with other studies 19, 20 showing a higher prevalence of invasive fungal diseases in males. Furthermore, the overwhelming majority of patients (97.9%) had diabetes, underscoring the role of diabetes as a significant risk factor for mucormycosis in the context of COVID-19.
Impact of corticosteroid therapy: All patients with mucormycosis received corticosteroid therapy as part of their COVID-19 treatment regimen. This finding is in concordance with multiple studies that have identified corticosteroid use as a major risk factor for mucormycosis in COVID-19 patients21.
Geographical and Temporal Trends: The study highlights the surge in mucormycosis cases during the delta variant wave of COVID-19 in India22. This geographical and temporal correlation indicated that specific strains of the virus may have a higher propensity to cause severe immunocompromised states conducive to fungal infections.
Conclusion
This study highlights the significant incidence of mucormycosis among COVID-19 patients, particularly those with uncontrolled diabetes and recent corticosteroid use. Identification of rare fungal species such as C .recurvatus and S .racemosum has expanded the known spectrum of pathogens that cause mucormycosis. The modified KOH-LPCB method is an effective and practical tool for rapid diagnosis of mucormycosis in resource-limited settings. Vaccination plays a crucial role in reducing the incidence of COVID-19 and its associated complications, including mucormycosis. Identifying rare fungal species emphasizes the need for comprehensive fungal diagnostics.
Acknowledgment
We like to acknowledge KAPV Government Medical College, Tiruchirapalli, Tamilnadu, India, and Sree Balaji Medical College and Hospital, Chrompet, Chennai, and technicians for their support.
Funding sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest
The author(s) do not have any conflict of interest.
Data Availability Statement
The manuscript incorporates all datasets produced or examined throughout this research study.
Ethics Statement
Ethical approved obtained from Institutional ethics committee.
Informed Consent
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials
Authors Contribution
Dr. Vyshnavee Subramaniyan, and Dr.Ezhilnilavan Murugesan planned the study and performed the necessary experiments in the laboratory, and Dr..Bindu prepared the manuscript. Dr.Mynooh Risha contributed to a part in preparing the manuscript. Dr.Bindu.D communicated the manuscript. All the authors have read and finalized the manuscript.
Reference
- Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, Huhndorf S, James T, Kirk PM, Lücking R, Thorsten Lumbsch H, Lutzoni F, Matheny PB, McLaughlin DJ, Powell MJ, Redhead S, Schoch CL, Spatafora JW, Stalpers JA, Vilgalys R, Aime MC, Aptroot A, Bauer R, Begerow D, Benny GL, Castlebury LA, Crous PW, Dai YC, Gams W, Geiser DM, Griffith GW, Gueidan C, Hawksworth DL, Hestmark G, Hosaka K, Humber RA, Hyde KD, Ironside JE, Kõljalg U, Kurtzman CP, Larsson KH, Lichtwardt R, Longcore J, Miadlikowska J, Miller A, Moncalvo JM, Mozley-Standridge S, Oberwinkler F, Parmasto E, Reeb V, Rogers JD, Roux C, Ryvarden L, Sampaio JP, Schüssler A, Sugiyama J, Thorn RG, Tibell L, Untereiner WA, Walker C, Wang Z, Weir A, Weiss M, White MM, Winka K, Yao YJ, Zhang N. A higher-level phylogenetic classification of the Fungi. Mycol Res. 2007;111(5):509-47.
CrossRef - Hoenigl M, Seidel D, Sprute R, Cunha C, Oliverio M, Goldman GH, Ibrahim AS, Carvalho A. COVID-19-associated fungal infections. Nat Microbiol. 2022;7(8):1127-1140.
CrossRef - Prakash H, Chakrabarti A. Epidemiology of mucormycosis in India. Microorganisms. 2021;4(3):523.
CrossRef - Pal R, Singh B, Bhadada S K, Banerjee M, Bhogal R S, Hage N, Kumar A.COVID-19-associated mucormycosis: An updated systematic review of the literature. Mycoses. 2021; 64(12):1452-1459.
CrossRef - Balushi AA, Ajmi AA, Sinani QA, Menon V, Berieki ZA, Shezawi AA, Azri SA, Rashdi AA, Jardani AA, Baluki TA, Ghaithi SA, Reesi AA, Al-Za’abi AT, Al’ Balushi MA, Maqbali TA. COVID-19-Associated Mucormycosis: An Opportunistic Fungal Infection. A Case Series and Review. Int J Infect Dis. 2022; 121:203-210.
CrossRef - Rao VUS, Arakeri G, Madikeri G, Shah A, Oeppen RS, Brennan P A. COVID-19 associated mucormycosis (CAM) in India: a formidable challenge. Br J Oral Maxillofac Surg.2021;59:1095–1098.
CrossRef - Sharma S, Grover M, Bhargava S, Samdani S, Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol Otol. 2021, 135(5):442-447.
CrossRef - Gupta R, Kesavadev J, Krishnan G, Agarwal S, Saboo B, Shah M, Mittal A, Durani S, Luthra A, Singhal A, Rasheed M, Rao GVS, Tripathi V, Jha A, Ghosh A, Mohan V, Singh AK, Phatak S, Panicker J, Bhadada SK, Joshi S, Pal R, Mithal A, Vikram N, Misra A. COVID-19 associated mucormycosis: A descriptive Multisite Study from India. Diabetes Metab Syndrome. 2021;5(6):102322.
CrossRef - Wondimu B, Bradley B, Lieberman JA, Cohen SA, Bui L, Reddi DM. Cokeromyces recurvatus Incidentally Found in a Patient with Gastric Outlet Obstruction. Mycopathologia, 2022;187:605-610.
CrossRef - Kemna ME, Neri R, Ali RA, Salkin IF. Cokeromyces recurvatus, a mucoraceous zygomycete rarely isolated in clinical laboratories. Journal of Clinical Microbiology.1994; 32:843-845.
CrossRef - Taghizadeh Armaki M, Jafarzadeh J, Mahdavi Omran S, Bayani M, Tavassoli A, Faeli L, Nosratabadi M, Yaalimadad S, Nikoueian B, Haghani I, Moazeni M, Shokohi T, Taghi Hedayati M, Abastabar M. First report of rhino-orbital mucormycosis caused by Syncephalastrum racemosum in a diabetic patient with COVID-19 in Iran and review of recent literature. Current Medical Mycology.2022; 8:49-54.
CrossRef - Verma S, Gupta PP, & Nain SA Rare Case of Invasive Pulmonary Infection by Syncephalastrum racemosum in a Pulmonary Tuberculosis Patient. The Indian Journal of Chest Diseases and Allied Sciences. 2023; 65(3):155-156.
CrossRef - Ribes JA, Vanover-Sams CL, and Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13(2):236-301.
CrossRef - Mohanty A, Gupta P, Arathi K, Rao S, Rohilla R, Meena S, Singh A, Kaistha N, Rath RS, Varshney S. Evaluation of Direct Examination, Culture, and Histopathology in the Diagnosis of Mucormycosis: Reiterating the Role of KOH Mount for Early Diagnosis. Cureus, 2021;13(11): e19455.
CrossRef - Hoenigl M, Seidel D, Carvalho A, Rudramurthy SM, Arastehfar A, Gangneux JP, Nasir N, Bonifaz A, Araiza J, Klimko N, Serris A, Lagrou K, Meis JF, Cornely OA, Perfect JR, White PL, Chakrabarti A; ECMM and ISHAM collaborators. The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries. Lancet Microbe.2022; 3(7):e543-e552.
CrossRef - Patel A, Agarwal R, Rudramurthy SM, Shevkani M, Xess I, Sharma R, Savio J, Sethuraman N, Madan S, Shastri P, Thangaraju D, Marak R, Tadepalli K, Savaj P, Sunavala A, Gupta N, Singhal T, Muthu V, Chakrabarti A; MucoCovi Network3. Multicenter Epidemiologic Study of Coronavirus Disease-Associated Mucormycosis, India. Emerg Infect Dis. 2021;27(9):2349-2359.
CrossRef - Seidel D, Simon M, Sprute R, Lubnow M, Evert K, Speer C, Seeßle J, Khatamzas E, Merle U, Behrens C, Blau IW, Enghard P, Haas CS, Steinmann J, Kurzai O, Cornely OA. Results from a national survey on COVID-19-associated mucormycosis in Germany: 13 patients from six tertiary hospitals. Mycoses. 2022; 65(1):103-109.
CrossRef - Sen M, Honavar SG, Bansal R, Sengupta S, Rao R, Kim U, Sharma M, Sachdev M, Grover AK, Surve A, Budharapu A, Ramadhin AK, Tripathi AK, Gupta A, Bhargava A, Sahu A, Khairnar A, Kochar A, Madhavani A, Shrivastava AK, Desai AK, Paul A, Ayyar A, Bhatnagar A, Singhal A, Nikose AS, Bhargava A, Tenagi AL, Kamble A, Nariani A, Patel B, Kashyap B, Dhawan B, Vohra B, Mandke C, Thrishulamurthy C, Sambare C, Sarkar D, Mankad DS, Maheshwari D, Lalwani D, Kanani D, Patel D, Manjandavida FP, Godhani F, Agarwal GA, Ravulaparthi G, Shilpa GV, Deshpande G, Thakkar H, Shah H, Ojha HR, Jani H, Gontia J, Mishrikotkar JP, Likhari K, Prajapati K, Porwal K, Koka K, Dharawat KS, Ramamurthy LB, Bhattacharyya M, Saini M, Christy MC, Das M, Hada M, Panchal M, Pandharpurkar M, Ali MO, Porwal M, Gangashetappa N, Mehrotra N, Bijlani N, Gajendragadkar N, Nagarkar NM, Modi P, Rewri P, Sao P, Patil PS, Giri P, Kapadia P, Yadav P, Bhagat P, Parekh R, Dyaberi R, Chauhan RS, Kaur R, Duvesh RK, Murthy R, Dandu RV, Kathiara R, Beri R, Pandit R, Rani RH, Gupta R, Pherwani R, Sapkal R, Mehta R, Tadepalli S, Fatima S, Karmarkar S, Patil SS, Shah S, Shah S, Shah S, Dubey S, Gandhi S, Kanakpur S, Mohan S, Bhomaj S, Kerkar S, Jariwala S, Sahu S, Tara S, Maru SK, Jhavar S, Sharma S, Gupta S, Kumari S, Das S, Menon S, Burkule S, Nisar SP, Kaliaperumal S, Rao S, Pakrasi S, Rathod S, Biradar SG, Kumar S, Dutt S, Bansal S, Ravani SA, Lohiya S, Ali Rizvi SW, Gokhale T, Lahane TP, Vukkadala T, Grover T, Bhesaniya T, Chawla U, Singh U, Une VL, Nandedkar V, Subramaniam V, Eswaran V, Chaudhry VN, Rangarajan V, Dehane V, Sahasrabudhe VM, Sowjanya Y, Tupkary Y, Phadke Y; members of the Collaborative OPAI-IJO Study on Mucormycosis in COVID-19 (COSMIC) Study Group. Epidemiology, clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India – Collaborative OPAI-IJO Study on Mucormycosis in COVID-19 (COSMIC), Report 1. Indian J Ophthalmol.2021; 69(7):1670-1692.
CrossRef - Egger M, Hoenigl M, Thompson GR 3rd, Carvalho A, Jenks JD. Let’s talk about sex characteristics-As a risk factor for invasive fungal diseases. Mycoses. 2022; 65(6):599-612.
CrossRef - Dam P, Cardoso MH, Mandal S, Franco OL, Sagirolu P, Polat OA, Kokoglu K, Mondal R, Mandal AK, Ocsoy I. Surge of mucormycosis during the COVID-19 pandemic. Travel Med Infect Dis. 2023;52:102557.
CrossRef - Gupta R, Kesavadev J, Krishnan G, Agarwal S, Saboo B, Shah M, Mittal A, Durani S, Luthra A, Singhal A, Rasheed M, Rao GVS, Tripathi V, Jha A, Ghosh A, Mohan V, Singh AK, Phatak S, Panicker J, Bhadada SK, Joshi S, Pal R, Mithal A, Vikram N, Misra A. COVID-19 associated mucormycosis: A Descriptive Multisite Study from India. Diabetes Metab Syndr. 2021;15(6):102322.
CrossRef - Rao VUS, Arakeri G, Madikeri G, Shah A, Oeppen RS, Brennan PA. COVID-19 associated mucormycosis (CAM) in India: a formidable challenge. Br J Oral Maxillofac Surg. 2021;59(9):1095-1098.
CrossRef







