Akanksha Singh Kachhawaha1 , Milda Elizabeth1
, Milda Elizabeth1 , Parvathy Hari1
, Parvathy Hari1 , Athulya Rajan2
, Athulya Rajan2 and Neelkant Verma2*
and Neelkant Verma2*
1School of Medico-Legal Studies, National Forensic Sciences University, Gandhinagar campus, Gujarat India.
2School of Forensic Sciences, National Forensic Sciences University, Gandhinagar campus, Gujarat India.
Corresponding Author E-mail:neelkant.verma@nfsu.ac.in
DOI : https://dx.doi.org/10.13005/bpj/3125
Abstract
Iron oxide nanoparticles (IONPs) synthesised via green methods hold immense promise for various applications, particularly in biomedicine and environmental remediation. In this study, we present a novel approach for the green synthesis of IONPs using butterfly pea flowers, Clitoria ternatea (CT), extracted as a reducing and stabilising agent. The physicochemical properties of the synthesised nanoparticles were extensively characterised through UV-visible spectrophotometry, revealing an absorption peak centred at 297 nm, and Dynamic Light Scattering (DLS) analysis resulted in a polydispersity index (PDI) of 0.227 and Z-average of 68.41 nm respectively, with a zeta potential value of -23.4 mV. Characterisation was performed using Scanning Electron Microscopy (SEM), Fourier Transform Infrared Spectroscopy (FTIR), and Energy Dispersive X-ray Fluorescence Spectrometry (EDXRF). Our results demonstrate the successful synthesis of IONPs, revealing their stability, morphology, elemental composition, and surface functionalization. Furthermore, the antibacterial activity of the synthesised IONPs against Escherichia coli (E. coli) was evaluated using the agar diffusion method, which showed inhibition of E. coli in a volume-dependent manner in the range 18-26 mm in comparison to 30 mm (positive control). These findings suggest the potential biomedical applications of these nanoparticles, particularly in antimicrobial therapy.
Keywords
Aparajita; Clitoria ternatea; Green Synthesis; IONPs
Download this article as:| Copy the following to cite this article: Kachhawaha A. S, Elizabeth M, Hari P, Rajan A, Verma N. Green Synthesis and Antibacterial Evaluation of Iron Oxide Nanoparticles Using Clitoria Ternatea Flowers. Biomed Pharmacol J 2025;18(1). |
| Copy the following to cite this URL: Kachhawaha A. S, Elizabeth M, Hari P, Rajan A, Verma N. Green Synthesis and Antibacterial Evaluation of Iron Oxide Nanoparticles Using Clitoria Ternatea Flowers. Biomed Pharmacol J 2025;18(1). Available from: https://bit.ly/4jU34ee |
Introduction
Nanoparticles of metal oxide are of significance because of their widespread use in medicine, material science, electronics, etc. Generally, green synthesis of nanoparticles does not employ any hazardous chemicals; hence, it has emerged as an important branch of nanotechnology which has attracted attention owing to its eco-friendly nature and potential applications in various fields, including biomedicine and environmental remediation1,2. Different biological materials such as plants, bacteria, fungi, etc. are used as sources for ‘green’ synthesis of metal and metal oxides NPs 3–5, where it has been revealed that plant-based green synthesis is much simpler and easier to use than bacteria/fungi mediated synthesis6.
For the green synthesis of nanoparticles, butterfly pea (Clitoria ternatea), a perennial herbaceous plant, is a promising plant owing to its rich phytochemical composition7. This plant is widely distributed across the tropical and subtropical regions. Clitoria ternatea also known as ‘Aparajita’ in the Indian Ayurveda system and used as a nerve tonic8. The plant has a wiry-look stem, the 2-4 cm blooms are surrounded by complex leaves with three to nine elliptical or oval leaflets. They are lengthy and contain various blue hues9. Plant roots have been used to treat several conditions, including constipation, arthritis, eye conditions, and indigestion. Additionally, the flower has been suggested for snake-bite therapy10 and used to cure abdominal visceral oedema, ascetics, skin issues, and sore throat. In addition to its antimicrobial, antiviral, anti-allergic, and anti-inflammatory properties, the flower also has the capacity to fight diabetes, safeguard the cardiovascular system, and offer a host of other health advantages11. Flavonoids, glycosides, anthocyanins, polyphenols, and flavonols detected in flowers are organic antioxidants12,13. CT flowers are particularly rich in bioactive compounds, such as flavonoids, alkaloids, and tannins, which possess inherent reducing and stabilising capabilities, making them ideal candidates for the green synthesis of nanoparticles14.
Iron oxide nanoparticles (IONPs) have gained immense interest in biomedical applications, particularly as antibacterial agents, because of their intrinsic magnetic properties that allow them to be readily retrieved from the reaction mixture by subjecting them to an external magnetic field. The antibacterial efficacy of IONPs is attributed to their ability to induce oxidative stress, disrupt bacterial membranes, and interfere with cellular processes15 making them a distinguished biocompatible nanoparticle. Moreover, surface functionalization of IONPs with natural compounds derived from plant extracts can enhance their stability, biocompatibility, and antimicrobial activity.
In this study, we present the green synthesis of iron oxide nanoparticles using Clitoria ternatea flowers as a reducing and stabilising agent. We aimed to investigate the physicochemical properties of the synthesised nanoparticles and evaluate their antibacterial activity.
Materials And Methods
Reagents and chemicals
Anhydrous Ferric Chloride (FeCl3) and Sodium Hydroxide (NaOH) were obtained from SRL India. Ethanol was obtained from Merck (Darmstadt, Germany). Hydrogen peroxide (H2O2) solution (10%) and DMSO were procured from HiMedia (Mumbai, India). Milli-Q water was obtained from a Lab Link Water System.
Preparation of Clitoria ternatea flower extract
In March, fresh flowers of Clitoria ternatea were harvested from various locations and regions of Gandhinagar, Gujarat. They were then meticulously washed with distilled water to eliminate extraneous matter. The flowers were then carefully placed in an airtight container and subjected to shade drying for a duration of three days to reduce their moisture content. Following desiccation, the flowers were finely ground into powder. A predetermined amount of this powder (5 g) was dissolved in 100 ml of distilled water to form an aqueous extract. To facilitate the extraction of bioactive compounds, the extract was heated in a water bath at 70 °C for 30 min. During this process, the flask containing the solution was shielded from light exposure by wrapping it with aluminum foil, thereby minimising the degradation of light-sensitive compounds. After heating, the extract was filtered using Whatman filter paper no. 41 to remove insoluble particulate matter, yielding a clear solution. Finally, the filtered extract was stored in a refrigerator maintained at 4°C. This methodology adhered to established scientific protocols for the extraction of phytochemicals from botanical sources13,16.
LCMS characterization of the flower extract
Analysis of the extract was performed using a combined system consisting of Shimadzu ultrafast liquid chromatography LC-30A and triple quadrupole mass spectrometer LCMS-8040. Separation was performed using a Shimpack GIST C18 column (4.6×150 mm, 5µm). The flow rate was maintained at 0.5 mL/min and the column temperature was 40°C. A gradient elution program was set with mobile phase A (aqueous solution with 0.1% v/v formic acid) and mobile phase B (methanol), where the time program proceeded from 0 to 3 min with solvent B linearly increasing from 0 to 40%; from 3 to 8 min, the percentage of solvent B linearly increased to 60%; from 8 to 10 min, solvent B percentage linearly increased to 90% and remained constant up to 14 min. From 14 to 14.5 min the concentration of B decreased to 0% and was maintained for up to 20 min. The injection volume was 20µL. After optimisation, the heat-block temperature was set to 350°C in positive-ion mode with a capillary voltage of 6 kV. Nitrogen gas was used as nebulizer gas and drying gas at flow rates of 2.8 L/min and 11 L/min respectively. Argon gas was used as collision gas. MRM mode was used for detection.
Green synthesis of iron oxide nanoparticles
Nanoparticles were synthesised following the method described by Bhuiyan17 with slight modifications. A freshly prepared solution of 0.1 M FeCl3 was added dropwise to 100 mL of the flower extract under gentle but continuous agitation. Subsequently, the pH of the solution was adjusted to 8 by using 1 M NaOH. The discernible change in the colour of the resultant solution from violet to black indicates the successful synthesis of iron-oxide nanoparticles16,18,19. The reaction mixture was agitated on a rotary shaker for 24 h, followed by centrifugation at 9500 rpm for 15 min. The resulting precipitate was thoroughly washed with deionised water and ethanol and subsequently air-dried in a hot-air oven at 80 °C for 1 h. The resultant material was finely ground using a mortar and pestle and stored for subsequent characterisation.
Characterization of the synthesized nanoparticles
Molecular spectroscopy was performed using a UV-visible spectrophotometer (Thermo Evolution 201) by scanning the aqueous dispersion of nanoparticles in the region of 200-800 nm. Dynamic Light Scattering (DLS) measurements were carried out to measure the polydispersity index, particle size, and zeta potential of the synthesised IONPs using a Zetasizer Nano S90 dynamic light scattering instrument (Malvern Instruments Ltd., UK). Twenty measurements were taken and averaged at a constant temperature of 25 °C. Data were processed using the Malvern Zetasizer Software 7.11. Before DLS characterisation, the nanoparticles were thoroughly diluted using Milli-Q water, sonicated for 15 min, and filtered using a 0.22µm membrane filter. FTIR was used to determine the feature peaks for iron oxide nanoparticles and is denoted as a percentage of transmittance (%) on the y-axis and as wavenumber (cm-¹) on the x-axis. FTIR spectrum acquired with a range of wavenumbers from 4000 to 400 cm–¹. SEM was performed to analyse the morphology of the IONPs using a Jeol JSM 6010 LA apparatus. A Shimadzu EDX-7000 was employed for Energy Dispersive X-ray Fluorescence Spectrometry (EDXRF), which allowed the determination of the composition of elements present in the synthesised IONPs20.
Evaluation of Antibacterial Activity
The antibacterial action of IONPs made from Clitoria ternatea flower extract was tested using the agar diffusion method. The bacterial Strains used for the evaluation were obtained from the Toxicology Laboratory, NFSU. The synthesised NPs were used to test the antibiotic susceptibility profile of Escherichia coli (E. coli OP 50 strain). Mueller Hinton Agar (MHA), was employed in the subculture of pure bacterial cultures21. The wells on the Mueller Hinton Agar (MHA) plates had a diameter of 10 mm. Sterile cotton swabs were used to uniformly distribute the strain over each plate. The plates were then covered with discs impregnated with 100µL, 200µL and 300µL of IONPs at a concentration of 10 mg/mL. After 24 h of incubation at 37 °C, the bacterial zone of inhibition was measured.
Results and Discussion
LCMS characterization of the flower extract
LC-MS/MS characterisation of the flower extract resulted in the detection of delphinidin-3-o-glucoside (m/z 303) and cyanidin 3-(6″-malonylglucoside) (m/z-287), consistent with previous reports22. The corresponding mass spectra are shown in Figure 1 and 2. Previous studies have suggested that these phytochemicals in the flower extract act as reducing and capping agents, leading to green synthesis of nanoparticles23,24.
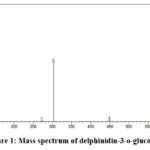 |
Figure 1: Mass spectrum of delphinidin-3-o-glucoside Click here to view Figure |
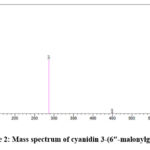 |
Figure 2: Mass spectrum of cyanidin 3-(6″-malonylglucoside)Click here to view Figure |
Synthesis of IONPs
In the synthesis, a combination of iron precursor FeCl3 and Clitoria ternatea flower extract served as the primary reaction mixture. A diverse array of compounds present in floral extracts, such as glycosides, polyphenols, tannins, and flavonoids, function as reducing and stabilising agents during the formation of nanoparticles (NPs)25. The formation of black-coloured precipitates arises from the interaction between phytochemicals and metal ions, thereby facilitating the formation of Fe₂O₃ nanoparticles17. The conversion of oxidation states, such as delphinidin and cyanidin derivatives, facilitated the conversion of oxidation states. These molecules possess strong antioxidant properties which contribute to the reduction of Fe0 and further oxidation of the metal core, leading to the formation of Fe2O3 nanoparticles26,27.
Characterisation of Iron Oxide Nanoparticles
UV-Vis Spectrophotometry
The formation of nanoparticles was initially inferred from the observable shift in solution colour to black and subsequently validated through analysis using a dual-beam UV-visible spectrophotometre. Figure. 3 presents the UV spectrum of Fe₂O₃ NPs, revealing an absorption peak centred at 297 nm. The synthesis of iron-oxide nanoparticles is facilitated by the dual roles of plant polyphenols and flavonoids, which serve as reducing and capping agents, respectively. Consequently, UV-Vis analysis indicated that the Fe₂O₃ nanoparticles exhibited a strong absorbance at ∼297 nm, suggesting the photosensitive nature of the synthesised particles within the UV spectral region28.
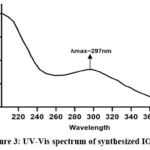 |
Figure 3: UV-Vis spectrum of synthesized IONPsClick here to view Figure |
Dynamic Light Scattering
Zeta potential and particle size experiments were conducted to evaluate the stability and hydrodynamic dimensions (size) of the synthesised iron oxide nanoparticles (IONPs). The mean hydrodynamic radii (r.nm) were employed to assess particle accumulation in aqueous media29. Our experimental findings yielded particles with a polydispersity index (PDI) and Z-average of 0.227 and 68.41 nm respectively (Figure. 4). Consistent findings from previous studies30 supported the negative potential values indicative of reasonably high stability. The particle size of IONPs and negative zeta potential of -23.4 mV indicate the formation of agglomerated and structurally stable nanoparticles. This formation may be attributed to the electrostatic forces, weak van der Waals interaction, and particles held by strong chemical bonds31. In our study, the stable IONPs exhibited a zeta potential of -23.4 mV (Figure. 5). This elevated negative potential value may be attributed to the capping of polyphenolic components present in plant extracts32. The negative zeta potential values also indicate their stability over prolonged periods in solution. The repulsive forces between negatively charged nanoparticles contribute to their stability and high dispersity within the solution.
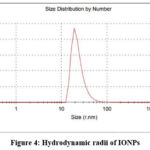 |
Figure 4: Hydrodynamic radii of IONPsClick here to view Figure |
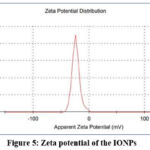 |
Figure 5: Zeta potential of the IONPsClick here to view Figure |
Scanning Electron Microscopy (SEM)
The structural analysis of the synthesized nanoparticles was performed using scanning electron microscopy (SEM). As shown in Figure.6, the SEM images depict the presence of iron oxide nanoparticles produced using Clitoria ternatea extract. The images reveal the formation of partially rhombic-shaped agglomerated IONPs. In a similar work, Farooq and co-workers reported rhombic-shaped IONPS using Corymbia citriodora33. Nanoparticle agglomeration could be attributed to several factors including the constituents of distinct bioactive reduction agents, the diminished capping efficacy of the floral extract, and the hydrogen bonding interactions associated with bioactive chemicals34. Agglomeration can be also observed in particles due to electrostatic interactions between layers of nanoparticle surfaces which was previously reported in a study35.
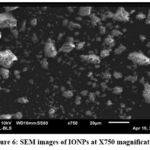 |
Figure 6: SEM images of IONPs at X750 magnificationClick here to view Figure |
FTIR
According to FTIR Spectrum, IONPs are synthesized by the bioactive substances found within the CT flower extract. The peaks at 2948 cm-1, 2902 cm-1, 2839 cm-1 are regarded as an indication of carboxylic acid, and the peaks at 3367 cm-1 and 3272 cm-1 suggest the stretching of the -OH bond from the aqueous phase. The peaks at 2902 cm-1, 2839 cm-1, and 2749 cm-1 additionally illustrate the stretching of the C-H group in the alkene group of the plant extract. The stretching of the C≡C bond of the alkyne is attributed to the peaks at 2188 cm-1, 2174 cm-1, 2146 cm-1, and 2111 cm-1. The stretching of the C-H bond of the aromatic group contained in the plant extract was demonstrated by the bands at 1864 cm-1, 1674 cm-1, and 1624 cm-1. The stretching of the N-O bond of nitro compounds, aromatic amines, the -OH bond of carboxylic acid, the C-O bond of aryl alkyl ether, and the alkyl halide are displayed by peaks at 1530 cm-1, 1463 cm-1, 1391 cm-1, 1275 cm-1, and 1251 cm-1. The C-O bond of esters could be observed in the peaks at 1180 cm-1 and 1118 cm-1. The C-N bond of aliphatic amines was demonstrated by the peak at 1052 cm-1. The primary and secondary amines are indicated by the peak at 869 cm-1. Alkene’s C=C bond has been demonstrated by the peak on 849 cm-1. Different varieties of iron oxide nanoparticles may be present in the bands at 699 cm-1, 632 cm-1, 611 cm-1, 527 cm-1, and 509 cm-1 (Figure. 7)36.
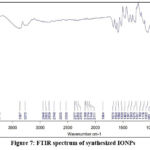 |
Figure 7: FTIR spectrum of synthesized IONPsClick here to view Figure |
Energy dispersive X-ray analysis
Energy Dispersive X-ray Spectroscopy (EDS) was employed to ascertain the elemental composition of Iron Oxide Nanoparticles (IONPs), revealing iron as a prominent metallic constituent. The presence of iron (Fe), chlorine (Cl), phosphorus (P), manganese (Mn), sulfur (S), calcium (Ca), potassium (K), and zinc (Zn) was confirmed by EDS analysis of the sample material, as shown in Table 1. Quantitative analysis, revealed that the EDX spectrum contained intense peaks of Fe (92.24%) and Cl (5.080%), in addition to minor peaks of P, Ca, S, Zn, K, and Mn. The Fe and Cl peaks may have originated from the FeCl3 precursors used in the fabrication of these nanoparticles. These minor peaks are likely attributable to the presence of polyphenols in the CT extract28. These observations offer valuable insights into the elemental composition of IONPs, emphasising the predominant presence of Fe along with trace constituents.
Table 1: Quantitative result of the elements present in IONPs.
| Quantitative Result | |||||
| Analyte | Result | Std.Dev. | Calc.Proc | Line | Intensity |
| Fe | 92.724 % | [0.124] | Quan-FP | FeKa | 2249.281 |
| Cl | 5.080 % | [0.113] | Quan-FP | ClKa | 6.8743 |
| P | 0.992 % | [0.083] | Quan-FP | P Ka | 0.1208 |
| Mn | 0.305 % | [0.006] | Quan-FP | MnKa | 7.1594 |
| S | 0.241 % | [0.029] | Quan-FP | S Ka | 0.1046 |
| Ca | 0.192 % | [0.009] | Quan-FP | CaKa | 0.3740 |
| Zn | 0.177 % | [0.006] | Quan-FP | ZnKa | 2.6561 |
| K | 0.121 % | [0.014] | Quan-FP | K Ka | 0.1331 |
| Sm | 0.114 % | [0.020] | Quan-FP | SmLa | 0.7490 |
| Cu | 0.055 % | [0.007] | Quan-FP | CuKa | 0.6938 |
| Li2B4O7 | 0.000 : 1 | [—–] | Flux | —— | —— |
| Li2B4O7 | 0.000 : 1 | [—–] | Flux | —— | —— |
Antibacterial Activity
E. coli was utilized to investigate the anti-bacterial efficacy of green synthesized IONP. The results showed that IONPs exhibited a higher zone of inhibition in a concentration dependent manner. The highest inhibition zone was observed as 26mm at 300µL as shown in Figure. 8(e). The measurements are shown in Table 2 and inhibition zones were measured by the Kirby-Bauer disk diffusion test37. These findings support the hypothesised mechanism of the antibacterial activity of FeNPs, which involves particle aggregation via the cytosol. The capacity of a nanoparticle to enter and accumulate inside the wall of a bacterial cell increases with decreasing size. Nanoparticles cause the bacterial cell membrane to break and the cellular content to leak out. The emergence of ROS, such as hydroxyl radicals and singlet oxygen, inside bacterial cells, is another theory for the antibacterial efficacy of FeNPs. The Fenton reaction between Fe and the metabolic products of bacterial cells causes ROS to form substances, such as hydrogen peroxide. Oxidative stress results in ROS production, which causes bacterial cell death. Although the antibacterial mechanism of action of FeNPs is unknown, it is known that these nanoparticles may serve as antimicrobial agents38.
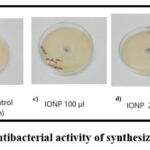 |
Figure 8: Antibacterial activity of synthesized IONPsClick here to view Figure |
Table 2: Zone of inhibition of IONPs synthesized from CT flower extract
| Bacteria | Zone of Inhibition (mm) | ||||
| 100 µL | 200 µL | 300 µL | Positive | Negative | |
| E.coli | 18mm | 20mm | 26mm | 30mm | – |
Conclusion
Iron oxide nanoparticles (IONPs) were effectively synthesised using a green synthesis approach with the flower extract of Clitoria ternatea. LC-MS characterisation studies were performed using UV-Vis spectrophotometry, EDXRF, FTIR, SEM, DLS, and a Zetasizer. The synthesised NPs have significant antibacterial efficacy at a concentration of 10 mg/ml and a volume of 300 µl on E. coli strain, making them potential for biomedical and pharmaceutical applications. This study reveals the versatility and efficiency of iron oxide nanoparticles as materials with numerous uses in science and technology. The findings of this study could have a substantial impact on the development of ecologically friendly and sustainable strategies for nanoparticle synthesis. Future research could concentrate on optimising the production method and investigating photocatalytic dye degradation using these nanoparticles. Furthermore, the anticancer activity of the synthesised nanoparticles can be investigated to assess their potential use in cancer treatment.
Acknowledgment
The authors would like to thank National Forensic Sciences University, Gandhinagar for providing the infrastructure, experimental facilities, and support that made this work possible.
Funding Source
The author(s) received no financial support for the research, authorship, and/or publication of this article
Conflict of Interest
The author(s) do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials.
Author contributions
All authors have contributed significantly to the overall project;
- Akanksha Singh Kachhawaha and Neelkant Verma: Concept and Methodology;
- Milda Elizabeth and Athulya Rajan: Investigation and Analysis,
- Parvathy Hari Visualization and artwork;
- Akanksha Singh Kachhawaha, Neelkant Verma and Parvathy Hari Editing and Reviewing manuscript draft.
- All authors discussed the results and contributed to the final manuscript.
References
- Soltys L, Olkhovyy O, Tatarchuk T, Naushad M. Green Synthesis of Metal and Metal Oxide Nanoparticles: Principles of Green Chemistry and Raw Materials. Magnetochemistry. 2021;7(11):145. doi:10.3390/magnetochemistry7110145
CrossRef - Akintelu SA, Folorunso AS, Folorunso FA, Oyebamiji AK. Green synthesis of copper oxide nanoparticles for biomedical application and environmental remediation. Heliyon. 2020;6(7):e04508. doi:10.1016/j.heliyon.2020.e04508
CrossRef - Abisharani JM, Devikala S, Kumar .R. Dinesh, Arthanareeswari M, Kamaraj P. Green synthesis of TiO2 Nanoparticles using Cucurbita pepo seeds extract. Mater Today Proc. 2019;14:302-307. doi:10.1016/j.matpr.2019.04.151
CrossRef - Abdelraof M, Hasanin MS, Farag MM, Ahmed HY. Green synthesis of bacterial cellulose/bioactive glass nanocomposites: Effect of glass nanoparticles on cellulose yield, biocompatibility and antimicrobial activity. Int J Biol Macromol. 2019;138:975-985. doi:10.1016/j.ijbiomac.2019.07.144
CrossRef - Santhosh PB, Genova J, Chamati H. Green Synthesis of Gold Nanoparticles: An Eco-Friendly Approach. Chemistry. 2022;4(2):345-369. doi:10.3390/chemistry4020026
CrossRef - Singh J, Dutta T, Kim KH, Rawat M, Samddar P, Kumar P. ‘Green’ synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J Nanobiotechnology. 2018;16(1):84. doi:10.1186/s12951-018-0408-4
CrossRef - Madhumitha H, Ranjani S, Karunyaa JR, Hemalatha S. Clitoria Ternatea Floral Mediated Synthesis, Characterization, Antioxidant, and Cytotoxicity Evaluation of Silver Nanoparticles: Clitoria ternatea floral mediated SNP to control BC. Arch Breast Cancer. Published online May 1, 2023:222-231. doi:10.32768/abc.2023103222-231
CrossRef - Mukherjee PK, Kumar V, Kumar NS, Heinrich M. The Ayurvedic medicine Clitoria ternatea–from traditional use to scientific assessment. J Ethnopharmacol. 2008;120(3):291-301. doi:10.1016/j.jep.2008.09.009
CrossRef - Multisona R, Shirodkar S, Arnold M, Gramza Michalowska A. Clitoria ternatea Flower and Its Bioactive Compounds: Potential Use as Microencapsulated Ingredient for Functional Foods. Appl Sci. 2023;13:2134. doi:10.3390/app13042134
CrossRef - Rout G. Effect of cytokinins and auxins on micropropagation of Clitoria ternatea L. Biol Lett. 2004;41.
CrossRef - Ghosh D, Konishi T. Anthocyanins and anthocyanin-rich extracts: role in diabetes and eye function. Asia Pac J Clin Nutr. 2007;16(2):200-208.
- Jamil N, Zairi M, Nasim A, Paee F. Influences of Environmental Conditions to Phytoconstituents in Clitoria ternatea (Butterfly Pea Flower) – A Review. J Sci Technol. 2018;10. doi:10.30880/jst.2018.10.02.029
CrossRef - Prabhu S, Thangaian DT, Bharathy PV. Green-based Biosynthesis of Zinc Oxide Nanoparticles Using Clitoria ternatea Flower Extract and Its Antibacterial Activity. Nano Biomed Eng. 2021;13(4):394-400. doi:10.5101/nbe.v13i4.p394-400
CrossRef - Khwannimit D, Maungchang R, Rattanakit P. Green synthesis of silver nanoparticles using Clitoria ternatea flower: an efficient catalyst for removal of methyl orange. Int J Environ Anal Chem. 2020;102. doi:10.1080/03067319.2020.1793974
CrossRef - Nadeem M, Khan R, Shah N, Bangash IR, Abbasi BH, Hano C, Liu C, Ullah S, Hashmi SS, Nadhman A, Celli J. A Review of Microbial Mediated Iron Nanoparticles (IONPs) and Its Biomedical Applications. Nanomaterials. 2021;12(1):130. doi:10.3390/nano12010130
CrossRef - Priya, Naveen, Kaur K, Sidhu AK. Green Synthesis: An Eco-friendly Route for the Synthesis of Iron Oxide Nanoparticles. Front Nanotechnol. 2021;3. doi:10.3389/fnano.2021.655062
CrossRef - Bhuiyan MdSH, Miah MY, Paul SC, Aka TD, Saha O, Rahaman MdM, Sharif MdJI, Habiba O, Ashaduzzaman Md. Green synthesis of iron oxide nanoparticle using Carica papaya leaf extract: application for photocatalytic degradation of remazol yellow RR dye and antibacterial activity. Heliyon. 2020;6(8):e04603. doi:10.1016/j.heliyon.2020.e04603
CrossRef - Prasad AS. Iron oxide nanoparticles synthesized by controlled bio-precipitation using leaf extract of Garlic Vine (Mansoa alliacea). Mater Sci Semicond Process. 2016;53:79-83. doi:10.1016/j.mssp.2016.06.009
CrossRef - Senthil M, Ramesh C. Biogenic synthesis of Fe3O34 nanoparticles using tridax procumbens leaf extract and its antibacterial activity on Pseudomonas aeruginosa. Dig J Nanomater Biostructures. 2012;7:1655-1661.
- Ajinkya N, Yu X, Kaithal P, Luo H, Somani P, Ramakrishna S. Magnetic Iron Oxide Nanoparticle (IONP) Synthesis to Applications: Present and Future. Materials. 2020; 13(20):4644. https://doi.org/10.3390/ma13204644
CrossRef - Hindler JF, Munro S, eds. Antimicrobial susceptibility testing. Clinical Microbiology Procedures Handbook. Published online August 2010. doi:10.1128/9781555817435.ch5
CrossRef - Thuy NM, Minh VQ, Ben TC, Thi Nguyen MT, Ha HTN, Tai NV. Identification of Anthocyanin Compounds in Butterfly Pea Flowers (Clitoria ternatea L.) by Ultra Performance Liquid Chromatography/Ultraviolet Coupled to Mass Spectrometry. Molecules. 2021;26(15):4539. doi:10.3390/molecules26154539
CrossRef - Fatimah I, Hidayat H, Nugroho B, Husein S. Ultrasound-assistedss biosynthesis of Silver and Gold Nanoparticles using Clitoria ternatea flower. South Afr J Chem Eng. 2020;34. doi:10.1016/j.sajce.2020.06.007
CrossRef - Vorobyova V, Vasyliev G, Skiba M. Eco-friendly “green” synthesis of silver nanoparticles with the black currant pomace extract and its antibacterial, electrochemical, and antioxidant activity. Appl Nanosci. 2020;10(12):4523-4534. doi:10.1007/s13204-020-01369-z
CrossRef - Rajakumar G, Rahuman AA, Roopan SM, Khanna VG, Elango G, Kamaraj C, Zahir AA, Velayutham K. Fungus-mediated biosynthesis and characterization of TiO2 nanoparticles and their activity against pathogenic bacteria. Spectrochim Acta A Mol Biomol Spectrosc. 2012;91:23-29. doi:10.1016/j.saa.2012.01.011
CrossRef - Zúñiga-Miranda J, Guerra J, Mueller A, Mayorga-Ramos A, Carrera-Pacheco SE, Barba-Ostria C, Heredia-Moya J, Guamán LP. Iron Oxide Nanoparticles: Green Synthesis and Their Antimicrobial Activity. Nanomater Basel Switz. 2023;13(22):2919. doi:10.3390/nano13222919
CrossRef - Selvaraj R, Pai S, Vinayagam R, Varadavenkatesan T, Kumar PS, Duc PA, Rangasamy G. A recent update on green synthesized iron and iron oxide nanoparticles for environmental applications. Chemosphere. 2022;308:136331. doi:10.1016/j.chemosphere.2022.136331
CrossRef - Kiwumulo HF, Muwonge H, Ibingira C, Lubwama M, Kirabira JB, Ssekitoleko RT. Green synthesis and characterization of iron-oxide nanoparticles using Moringa oleifera: a potential protocol for use in low and middle income countries. BMC Res Notes. 2022;15(1):149. doi:10.1186/s13104-022-06039-7
CrossRef - Kumar SS, Venkateswarlu P, Rao VR, Rao GN. Synthesis, characterization and optical properties of zinc oxide nanoparticles. Int Nano Lett. 2013;3(1):30. doi:10.1186/2228-5326-3-30
CrossRef - Bharathi D, Bhuvaneshwari V. Synthesis of zinc oxide nanoparticles (ZnO NPs) using pure bioflavonoid rutin and their biomedical applications: antibacterial, antioxidant and cytotoxic activities. Res Chem Intermed. 2019;45(4):2065-2078. doi:10.1007/s11164-018-03717-9
CrossRef - Khan M, Ware P, Shimpi N. Synthesis of ZnO nanoparticles using peels of Passiflora foetida and study of its activity as an efficient catalyst for the degradation of hazardous organic dye. SN Appl Sci. 2021;3(5):528. doi:10.1007/s42452-021-04436-4
CrossRef - Park K, Tuttle G, Sinche F, Harper SL. Stability of citrate-capped silver nanoparticles in exposure media and their effects on the development of embryonic zebrafish (Danio rerio). Arch Pharm Res. 2013;36(1):125-133. doi:10.1007/s12272-013-0005-x
CrossRef - Panikar S, A. U, Umapathy D, Sumathi J, Mukherjee A, Prakash P, Farooq T. Morphological, chemoprofile and soil analysis comparison of Corymbia citriodora (Hook.) K.D. Hill and L.A.S. Johnson along with the green synthesis of iron oxide nanoparticles. J King Saud Univ – Sci. 2022;34:102081. doi:10.1016/j.jksus.2022.102081
CrossRef - Rao NH, N. L, Pammi SVN, Kollu P, S. G, P. L. Green synthesis of silver nanoparticles using methanolic root extracts of Diospyros paniculata and their antimicrobial activities. Mater Sci Eng C. 2016;62:553-557. doi:10.1016/j.msec.2016.01.072
CrossRef - Saqib S, Farooq M, Munis H, Zaman W, Ullah F, Syed, Shah N, Ayaz A, Bahadur S, Kashmir. Synthesis, characterization and use of iron oxide nano particles for antibacterial activity. Microsc Res Tech. 2019;82(4):415-420. doi:10.1002/jemt.23182.
CrossRef - Buarki F, AbuHassan H, Al Hannan F, Henari FZ. Green Synthesis of Iron Oxide Nanoparticles Using Hibiscus rosa sinensis Flowers and Their Antibacterial Activity. J Nanotechnol. 2022;2022:e5474645. doi:10.1155/2022/5474645
CrossRef - Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45(4):493-496.
CrossRef - Ramesh M, Anbuvannan M, Viruthagiri G. Green synthesis of ZnO nanoparticles using Solanum nigrum leaf extract and their antibacterial activity. Spectrochim Acta A Mol Biomol Spectrosc. 2015;136:864-870. doi:10.1016/j.saa.2014.09.105
CrossRef







