Reem Kalakattawi1 , Lena Gowharji2
, Lena Gowharji2 , Alaa marzogi3
, Alaa marzogi3 and Tahani Alghamdi4
and Tahani Alghamdi4
1Department of Breast imaging, Alhada armed forces hospital, Taif Saudi Arabia
2Department of Diagnostic Radiology, King Abdulaziz University Hospital, Jeddah, Saudi Arabia
3Department of Breast imaging, King Abdullah medical City, Makkah, Saudi Arabia
4Department of Radiology, consultant radiologist, King Abdullah Medical City, Makkah, Kingdom of Saudi Arabia
Corresponing Author E-mail:Dr.reem.m.k@hotmail.com
DOI : https://dx.doi.org/10.13005/bpj/3102
Abstract
This systematic review evaluates the diagnostic effectiveness of breast-specific gamma imaging (BSGI), MRI, mammography, and ultrasound in detecting breast lesions and their associations with molecular subtypes. Following PRISMA guidelines, a comprehensive search of PubMed, Embase, and Cochrane databases was conducted for studies published between January 2014 and October 2024. Nine studies met the inclusion criteria, encompassing clinical trials and observational studies that provided data on sensitivity, specificity, and diagnostic accuracy. Methodological quality was assessed using the QUADAS-2 tool, and pooled diagnostic measures were calculated with STATA V.14.0. BSGI demonstrated high diagnostic performance, with a sensitivity of 91.7% and specificity of 80.7%, surpassing mammography (77.3% sensitivity, 74.5% specificity) and ultrasound (82.1% sensitivity, 70.8% specificity). MRI exhibited the highest sensitivity at 92.5% but lower specificity at 69.7%. The diagnostic odds ratio for BSGI was 4.90 (95% CI: 3.12–7.68), emphasizing its role as a valuable adjunct to mammography and MRI, particularly in dense breast tissues and cases of inconclusive findings. The findings highlight the potential of BSGI to improve diagnostic accuracy and assist in identifying molecular subtypes, such as HER2-positive and triple-negative cancers, facilitating more personalized breast cancer treatment strategies. BSGI’s integration into diagnostic workflows offers promising advancements for breast cancer management.
Keywords
Breast Cancer Diagnosis; Breast-Specific Gamma Imaging; Mammography; Molecular Subtypes; MRI; Ultrasound
Download this article as:| Copy the following to cite this article: Kalakattawi R, Gowharji L, Marzogi A, Alghamdi T. Evaluating Diagnostic Technologies for Breast Lesions: A Focused Review of Breast-Specific Gamma Imaging, Magnetic Resonance Imaging, Mammography, and Ultrasound with Molecular Subtype Insights. Biomed Pharmacol J 2025;18(1). |
| Copy the following to cite this URL: Kalakattawi R, Gowharji L, Marzogi A, Alghamdi T. Evaluating Diagnostic Technologies for Breast Lesions: A Focused Review of Breast-Specific Gamma Imaging, Magnetic Resonance Imaging, Mammography, and Ultrasound with Molecular Subtype Insights. Biomed Pharmacol J 2025;18(1). Available from: https://bit.ly/42HzrGH |
Introduction
Breast cancer is a leading cause of morbidity and mortality among women globally, necessitating practical diagnostic approaches to ensure early detection and treatment.1 Among the various imaging modalities, mammography has traditionally been the most common screening tool due to its widespread availability and established role in early breast cancer detection.2 However, mammography is less effective in women with dense breast tissue, as dense tissue can obscure lesions, reducing sensitivity.3 This limitation has driven the development of additional imaging techniques, such as ultrasound, MRI, and Breast-Specific Gamma Imaging (BSGI), which offer complementary diagnostic capabilities.4
To address these limitations, advanced imaging techniques such as ultrasound, MRI, and Breast-Specific Gamma Imaging (BSGI) have been developed to enhance diagnostic accuracy. Each of these modalities offers unique advantages1. Ultrasound serves as a valuable follow-up tool, particularly effective in detecting abnormalities in dense breast tissues, but it is often operator-dependent. MRI provides high sensitivity, making it useful in high-risk populations, though its high cost and time requirements limit routine use. Meanwhile, BSGI is emerging as a promising adjunctive modality, leveraging functional imaging to detect metabolic activity associated with malignancies, which can help confirm findings from mammography or ultrasound. These advancements highlight the ongoing evolution of breast imaging to overcome the inherent challenges posed by dense breast tissue and improve overall diagnostic outcomes3.
Ultrasound, often used as a follow-up to mammography, is more effective in detecting lesions in dense breasts but is operator-dependent and may produce false positives.3 MRI, on the other hand, is highly sensitive for detecting breast lesions and is particularly useful in high-risk patients. Still, its high cost and longer examination time can limit its use in routine screening.1 In contrast, BSGI is emerging as a promising adjunctive imaging tool, particularly in cases where mammography and ultrasound yield inconclusive results.5 BSGI works by detecting areas of increased metabolic activity in breast tissue, characteristic of malignancies.6
Recent studies have demonstrated that BSGI offers higher specificity than both mammography and ultrasound, particularly in women with dense breasts, making it a valuable complement in breast cancer diagnostics.7 A meta-analysis comparing BSGI to other imaging modalities found that BSGI provided superior diagnostic accuracy in detecting breast lesions when combined with mammography and ultrasound.3 Furthermore, BSGI has shown promise in identifying specific molecular subtypes of breast cancer, offering a potential advantage in personalized treatment planning.1
MRI, although highly sensitive, has been shown to overestimate the extent of disease in some cases, leading to unnecessary biopsies or overtreatment.4 This issue can be mitigated using BSGI combined with MRI, as BSGI can help differentiate between malignant and benign lesions based on their metabolic activity.2 The combination of BSGI and MRI has proven particularly effective in evaluating residual tumor status following neoadjuvant chemotherapy, helping clinicians make informed decisions about subsequent treatments.4
The integration of BSGI into breast cancer diagnostics has been incredibly beneficial in patients with inconclusive findings from traditional imaging modalities.2 It is considered a cost-effective problem-solving strategy that reduces the need for unnecessary biopsies and follow-up imaging.6 Moreover, BSGI has demonstrated high sensitivity and specificity in detecting small breast lesions, which are often missed by mammography, particularly in dense breast tissues.8 This makes it a critical tool for comprehensive breast cancer diagnostics.
The correlation between imaging findings and molecular subtypes of breast cancer is a growing area of research, as molecular characteristics of tumors can significantly influence treatment outcomes.1 BSGI has shown potential in identifying specific subtypes of breast cancer, such as HER2-positive or triple-negative cancers, which tend to exhibit higher metabolic activity detectable by gamma imaging.5 This ability to correlate imaging results with molecular subtypes may allow for more targeted and individualized treatment strategies for breast cancer patients.3
In conclusion, while mammography and ultrasound remain standard tools in breast cancer screening, advanced imaging modalities like BSGI and MRI offer significant advantages, particularly in challenging cases involving dense breast tissue or inconclusive findings.7 Combining these techniques improves diagnostic accuracy and provides valuable insights into the molecular characteristics of breast lesions, paving the way for more personalized approaches to breast cancer management.4 As research continues, the role of BSGI in breast cancer diagnostics is expected to expand, offering clinicians a powerful tool for improving patient outcomes.5
In this study, the authors contributed collectively to conceptualizing and conducting the systematic review. Specifically, Reem Kalakattawi led the conceptualization and methodology design, Lena Gowharji and Alaa Marzogi conducted data collection and analysis, and Tahani Alghamdi supervised the project and provided critical revisions. The article is structured as follows: Section 2 outlines the materials and methods, detailing the search strategy, inclusion criteria, and statistical approaches. Section 3 presents the results, including key findings on diagnostic accuracy and comparisons among imaging modalities. Section 4 discusses the implications of these findings, with a focus on clinical applications and correlations with molecular subtypes. Finally, Section 5 concludes the study by summarizing its contributions and providing recommendations for future research.
The rationale of this Review
The rationale for conducting this systematic Review lies in the need for a comprehensive comparison of various imaging modalities used for the diagnosis of breast lesions, particularly about their ability to detect specific molecular subtypes of breast cancer. Breast cancer diagnostics rely heavily on imaging tools such as mammography, ultrasound, MRI, and, more recently, Breast-Specific Gamma Imaging (BSGI). While these modalities have individually proven effective, there is a lack of consolidated evidence on how each performs regarding molecular subtypes of breast cancer, which are critical for guiding treatment decisions.1 Given the advances in breast imaging technologies and their varying capabilities, it is essential to evaluate their accuracy, sensitivity, and specificity in diagnosing diverse breast lesions and correlating them with molecular characteristics.3
BSGI, in particular, is gaining attention for its potential to detect metabolically active lesions that might be missed by conventional imaging in dense breast tissue.5 Dense breast tissue can obscure lesions, reducing the efficacy of mammography, which remains the standard screening tool. The increasing use of BSGI as an adjunct imaging modality raises questions about its comparative value when used alongside or instead of ultrasound, mammography, and MRI, especially about different breast cancer subtypes, such as HER2-positive and triple-negative cancers.7 This systematic Review aims to fill the gap by comparing these imaging modalities and their diagnostic performance.
Another critical reason for this Review is the growing focus on personalized medicine, where accurate identification of tumor subtypes can influence treatment strategies.4 As breast cancer treatment becomes increasingly targeted, understanding the strengths and limitations of each imaging modality about specific molecular markers can aid clinicians in making more informed decisions. This systematic review seeks to synthesize current evidence from studies comparing BSGI, MRI, mammography, and ultrasound, focusing on their diagnostic accuracy regarding molecular subtypes. The findings will improve diagnostic strategies and potentially reduce unnecessary interventions, such as biopsies, by better matching imaging tools to patient-specific conditions.6
Development of the PICO Question:
The PICO framework is integral to structuring research questions in systematic reviews as it provides a clear and focused approach to defining the key components of the study. In the context of breast cancer diagnostics, the PICO framework ensures that the research question addresses the most critical aspects—population, intervention, comparison, and outcome—allowing for a systematic evaluation of the evidence. By explicitly identifying the population, such as women with suspected breast lesions, researchers can target studies that are highly relevant to the clinical context. This focus not only ensures that the results are applicable to real-world scenarios but also helps clinicians make evidence-based decisions tailored to patient needs.
The significance of PICO also lies in its ability to standardize the evaluation process across diverse imaging modalities, such as BSGI, MRI, mammography, and ultrasound. By clearly defining the intervention and comparison, PICO facilitates the identification of gaps in current diagnostic practices and highlights the added value of emerging tools like BSGI. Furthermore, emphasizing specific outcomes, such as the correlation between imaging findings and molecular subtypes, enhances the clinical relevance of the review. This targeted approach allows researchers to generate robust conclusions that directly inform clinical guidelines and improve patient care by ensuring that the best diagnostic strategies are adopted for specific breast cancer subtypes.
The PICO framework was chosen to structure the research question to ensure clarity and focus on the critical aspects of the systematic Review. The Population (P) in this context refers to women who present with suspected breast lesions, a common demographic requiring diagnostic imaging for early detection and proper classification of breast cancer. This population is significant because breast cancer is highly prevalent among women, and accurate diagnosis is crucial for effective treatment planning.
The Intervention (I) under investigation is Breast-Specific Gamma Imaging (BSGI), a relatively new imaging modality gaining attention for its ability to detect metabolically active lesions. BSGI is increasingly used as an adjunct tool for breast cancer diagnostics, particularly in patients with dense breast tissue, where traditional imaging modalities like mammography may not be as effective.7 The growing interest in BSGI warrants a detailed investigation of its performance compared to other commonly used imaging methods.
The Comparison (C) includes MRI, mammography, and ultrasound, which are well-established breast cancer diagnostics tools. Each of these modalities has its strengths and limitations, with mammography being the standard for routine screening, ultrasound often used as a follow-up, and MRI providing high sensitivity for detecting breast lesions. By comparing BSGI to these tools, this Review aims to evaluate the added value of BSGI, particularly in cases where the standard tools fall short, such as in dense breast tissues or in the differentiation of specific molecular subtypes of breast cancer.3
The Outcome (O) focuses on the diagnostic accuracy of these imaging modalities, specifically regarding their ability to detect breast lesions and correlate with molecular subtypes like HER2-positive, triple-negative, or luminal types. The correlation between imaging findings and molecular subtypes is critical, as molecular characteristics are increasingly used to guide personalized treatment approaches.4 Therefore, this systematic Review aims to identify which imaging method provides the most accurate and reliable results for specific breast cancer subtypes.
The research question for this systematic review, structured using the PICO framework, is as follows:
In women with suspected breast lesions (Population), how does Breast-Specific Gamma Imaging (Intervention) compare to MRI, mammography, and ultrasound (Comparison) in terms of diagnostic accuracy, including sensitivity, specificity, and correlation with molecular subtypes of breast cancer (Outcome)?
Materials and Methods
As the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommended, we conducted our study following the PRISMA specifications to ensure a transparent, systematic, and replicable process. Each step of the review process, from study selection to data extraction and synthesis, was performed according to PRISMA guidelines to maintain methodological rigor and minimize bias in reporting the findings.5 The following sections outline the key stages of the systematic Review, including literature search strategy, inclusion and exclusion criteria, data extraction, and quality assessment of the studies.
Search Strategy
To evaluate the diagnostic accuracy of BSGI, MRI, mammography, and ultrasound in diagnosing breast lesions and their correlation with specific molecular subtypes, we retrieved studies from the following databases: “PubMed, Embase, and the Cochrane Library”. Two reviewers, independently searched these databases for articles published between January 2014 and October 2024. Our search terms included “Breast-Specific Gamma Imaging”, “BSGI”, “Magnetic Resonance Imaging”, “MRI”, “Mammography”, “Ultrasonography”, “Breast Neoplasms”, “Breast Neoplasm”, “Breast Tumor”, “Breast Tumors”, “Breast Cancer”, “Malignant Neoplasm of Breast”, “Breast Malignant Neoplasm”, “Breast Carcinomas”, “Breast Carcinoma”, “breast mass”, “breast lesion”, “breast lesions”, “breast diseases”, and “molecular subtypes”. In addition, we reviewed the references of all included studies to identify further relevant research.
To assess the diagnostic effectiveness of BSGI, MRI, mammography, and ultrasound in detecting breast lesions and their association with specific molecular subtypes, a comprehensive literature search was conducted. The search strategy involved exploring a wide range of peer-reviewed articles published within the last decade, focusing on studies that evaluated these imaging modalities. Two reviewers independently carried out the search and study selection process to ensure objectivity and minimize bias. The search also included reviewing citations from relevant articles to identify additional studies that aligned with the review’s objectives.
This thorough approach was designed to ensure the inclusion of studies that provided valuable insights into the diagnostic accuracy of breast imaging techniques. By adopting a systematic and meticulous process, the review aimed to capture high-quality evidence that highlights the comparative performance of these modalities in diagnosing breast lesions and correlating findings with molecular subtypes. The independent review process and cross-referencing of citations further strengthened the reliability and scope of the study selection.
To ensure a comprehensive and unbiased search, we employed Boolean operators and combined various keywords to maximize the retrieval of relevant studies. This included using “AND” and “OR” to connect search terms effectively, allowing us to target studies addressing breast imaging and molecular subtype correlations. Furthermore, database filters were applied to focus on peer-reviewed journal articles, ensuring the inclusion of high-quality research. The inclusion of a decade-long publication range was intentional, capturing recent advancements and maintaining the relevancy of findings in this rapidly evolving field of diagnostic imaging. Our iterative search process also involved refining keywords based on initial results to identify additional studies that may have been overlooked.
Recognizing the potential limitations of database-only searches, we extended our scope by conducting a thorough manual search of reference lists from included articles. This step was critical in identifying studies that might not have been indexed with the chosen keywords or that were published in less commonly used journals. Additionally, we sought to include studies across various geographical regions to account for potential differences in imaging practices and technology access. This comprehensive approach ensured a robust dataset, providing a well-rounded evaluation of the diagnostic modalities in question. The involvement of two independent reviewers in the search and selection process minimized the risk of bias and enhanced the reliability of the results.
Inclusion and Exclusion Criteria
The inclusion criteria for this systematic Review were studies that focused on the diagnostic accuracy of Breast-Specific Gamma Imaging (BSGI), MRI, mammography, and ultrasound in detecting breast lesions, specifically emphasising their correlation with molecular subtypes of breast cancer. We included studies published between January 2014 and October 2024, written in English, involving human participants, and providing quantitative data on these imaging modalities’ sensitivity, specificity, and diagnostic performance. Studies that included comparisons between the imaging techniques in patients with suspected or confirmed breast cancer were also eligible.
The exclusion criteria were studies that did not provide sufficient diagnostic accuracy data or focused on imaging techniques unrelated to breast lesion detection. We excluded case reports, reviews, conference abstracts, editorials, and studies that involved non-human subjects or were unavailable in English. Additionally, studies involving imaging modalities irrelevant to this Review or without a direct comparison between BSGI, MRI, mammography, and ultrasound were excluded.
Study Screening
Two reviewers independently examined the titles and abstracts of articles obtained from the electronic databases, adhering to the pre-established inclusion and exclusion criteria. Each reviewer conducted a double screening of the studies to reduce the likelihood of errors or bias. When disagreements arose about the eligibility of a particular study, a third reviewer was involved to reach a final consensus. Full-text articles of studies that met the initial criteria were subsequently obtained for an in-depth review.
To prevent redundancy, studies authored by the same researchers or originating from the same institution were carefully evaluated. In such cases, only the most recent study with the largest sample size was selected. When different authors from the same institution were involved, we contacted the corresponding authors via email to clarify any overlap. If no response was received, the selection defaulted to the most recent and comprehensive study. This meticulous approach ensured the inclusion of only the most relevant and high-quality studies in the systematic review.
The systematic approach of involving multiple reviewers and consulting a third party in cases of disagreement was designed to enhance the objectivity and rigor of the study selection process. By having two reviewers independently evaluate each title and abstract, we minimized the influence of individual biases and ensured that decisions were rooted in the predefined inclusion and exclusion criteria. The practice of double screening by each reviewer further strengthened the reliability of the selection process, reducing the likelihood of missing eligible studies. This redundancy allowed for a cross-checking mechanism, ensuring that even borderline cases were carefully examined and deliberated upon. Consulting a third reviewer in cases of disagreement provided an additional layer of impartiality, guaranteeing that all decisions were balanced and fair.
Rationalizing the exclusion of duplicate studies was equally critical in maintaining the integrity of the systematic review. Selecting the most recent studies with the largest sample sizes ensured that the included data was both current and statistically robust, providing the most accurate reflection of the diagnostic modalities under review. Contacting corresponding authors in cases of institutional overlap helped clarify potential duplications and ensured that the selection process was transparent and inclusive. When authors could not be reached, prioritizing the most comprehensive studies allowed the review to proceed without compromising the quality of evidence. These strategies collectively contributed to the rigor and reliability of the systematic review, ensuring that the findings were based on the most relevant, high-quality, and non-redundant research available.
Data Abstraction
Two independent reviewers carried out data extraction from the chosen studies, ensuring a systematic and unbiased process. Any disagreements or inconsistencies in the extracted information were addressed and resolved through discussions involving a third reviewer. Key details were gathered from each study, specially the diagnostic outcomes, categorized as true or false positive or negative.
This step was significant for maintaining the accuracy and reliability of the data included in the review. By employing two independent reviewers, the process minimized the likelihood of errors or subjective interpretation during data extraction. This dual-review method ensured that critical details from the studies were captured comprehensively and consistently. Additionally, involving a third reviewer to resolve discrepancies introduced an impartial perspective, enhancing the credibility of the extracted data and the overall quality of the systematic review.
The meticulous extraction of specific study attributes, such as participant demographics and diagnostic outcomes, was essential for enabling meaningful comparisons across studies. The diagnostic categories (TP, FP, FN, and TN) provided the foundation for calculating performance metrics such as sensitivity, specificity, and predictive values, which were crucial for evaluating the diagnostic accuracy of the imaging modalities. This step not only ensured consistency in data reporting but also strengthened the analytical validity of the review, enabling robust and reproducible conclusions to be drawn.
Additionally, we collected data on the imaging modalities used, the specific molecular subtypes of breast cancer diagnosed, and each modality’s sensitivity, specificity, and overall diagnostic accuracy. Where available, we also extracted information on the study design, the imaging protocols followed, and any adjunctive imaging techniques employed. This comprehensive data collection ensured that the diagnostic performance of each imaging method was thoroughly evaluated and comparable across studies.
Quality Assessment
The quality of the included studies was assessed using QUADAS-2 framework. The tool examines potential sources of bias and issues related to applicability across four primary areas: selection of participants, the diagnostic test under evaluation, the reference standard used for comparison, and the sequence and timing of study procedures. To maintain consistency and impartiality, two reviewers independently performed the assessments. Any disagreements or discrepancies between their evaluations were addressed through collaborative discussions, with input from a third reviewer to reach a consensus and ensure accuracy.
This step was critical to the systematic review as it ensured the reliability and validity of the findings by focusing on the methodological rigor of the included studies. The QUADAS-2 tool provided a standardized framework to identify and minimize potential biases, such as selection bias or misclassification errors, which could compromise the quality of the review. By independently assessing each study, the process mitigated individual reviewer bias, while discussions involving a third reviewer added an additional layer of scrutiny and objectivity.
Assessing the risk of bias and applicability also highlighted any limitations in the studies, such as restricted generalizability due to patient selection or inconsistencies in the application of reference standards. Identifying these issues helped contextualize the findings, allowing for a more nuanced interpretation of the results. By ensuring that high-quality studies were emphasized in the analysis, this step enhanced the credibility of the conclusions and strengthened the evidence base for evaluating the diagnostic accuracy of the imaging modalities under investigation.
For each domain, we assessed the risk of bias as either low, high, or unclear. The patient selection domain was evaluated to ensure that studies avoided inappropriate exclusions and employed consecutive or random sampling. The index test domain assessed whether the imaging modalities (BSGI, MRI, mammography, and ultrasound) were interpreted without knowledge of the reference standard results. The reference standard domain evaluated whether the reference standard used for diagnosis (e.g., histopathology) was appropriate and applied consistently across the studies. Lastly, the flow and timing domain ensured that all patients received the same reference standard and that there were no significant time delays between the index test and the reference standard. Studies that met all criteria with a low risk of bias were considered high quality, while those with high or unclear risks in one or more domains were considered to have potential bias.
Statistical Analysis
Statistical analysis was conducted using STATA V.14.0 for all computations. The I² statistic was used to assess and quantify the heterogeneity between the included studies. A fixed-effect model was applied to consolidate the data if statistical heterogeneity was minimal or absent. Conversely, if heterogeneity was significant, a random-effect model was used to summarize the data.
The sensitivity for each imaging method was determined using the formula TP/(TP+FN), where TP denotes true-positive results and FN denotes false-negative results. Specificity was calculated as TN/(TN+FP), with TN representing true-negative results and FP representing false-positive results. Furthermore, additional diagnostic performance metrics, such as the positive likelihood ratio (LR+), negative likelihood ratio (LR-), and diagnostic odds ratio (DOR), were also calculated.
Results
Study Characteristics
After conducting a comprehensive search, a total of 12 studies were ultimately included in this systematic review. The detailed process of study selection is presented in the PRISMA flowchart (Figure 1). Out of 316 studies initially screened, 71 were chosen for full-text review. Following a thorough evaluation, nine studies met the inclusion criteria, as summarized in Table 1. These studies were published between 2014 and 2024 and involved patients who underwent diagnostic procedures using BSGI, MRI, mammography, and ultrasound prior to their breast lesion diagnosis and treatment. Furthermore, most of the included studies examined the relationship between imaging results and specific molecular subtypes of breast cancer (Figure 1).
This meticulous selection process highlights the focus on ensuring the relevance and quality of the included studies. By screening over 300 studies, the review aimed to capture a broad spectrum of research addressing breast imaging modalities. Narrowing this pool to nine high-quality studies ensured that the findings were based on robust and well-documented evidence. The inclusion of studies covering multiple imaging techniques allowed for a comparative analysis of their diagnostic capabilities, with an emphasis on their ability to detect breast cancer subtypes like HER2-positive and triple-negative cancers. This focus provides valuable insights into how imaging modalities can support personalized treatment planning (Figure 1).
Moreover, the studies selected for this review represent a range of patient demographics and imaging contexts, enhancing the generalizability of the findings. Evaluating imaging techniques in relation to molecular subtypes is particularly significant, as these subtypes influence treatment decisions and prognostic outcomes. By including studies that correlated imaging results with molecular characteristics, this review addresses an important gap in breast cancer diagnostics, offering clinicians evidence-based guidance for optimizing diagnostic accuracy and tailoring treatment strategies (Figure 1).
The included studies varied in design, representing both retrospective and prospective studies. Most patients in these studies were diagnosed with either malignant or benign breast lesions, and several studies specifically analyzed dense breast tissue, where mammography alone often proves insufficient. A significant proportion of patients across these studies were subjected to multiple imaging modalities, allowing for a robust comparison of diagnostic performance among BSGI, MRI, mammography, and ultrasound (Figure 1).
Furthermore, the methodological quality assessment of all included studies was performed using the QUADAS-2 tool, as shown in Supplementary Table 1. All studies were evaluated for risk of bias and applicability, and most were found to have a low risk of bias, with only minor concerns in a few studies. This thorough assessment ensured the reliability and accuracy of the findings in this systematic Review (Figure 1).
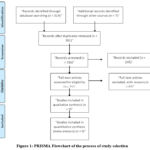 |
Figure 1: PRISMA Flowchart of the process of study selection Click here to view Figure |
Table 1: Characteristics of Included Studies Comparing BSGI, MRI, Mammography, and Ultrasound for Breast Cancer Diagnosis
| Author | Year | Patients | Lesions | BC (Breast Cancer) | MRI | BSGI | Mammography | Ultrasound | Median Age | Contrast Media | Country |
| Liu4 | 2021 | 390 | 229 | 229 | Yes | Yes | Yes | Yes | 56 | No | China |
| Kim7 | 2023 | 548 | 628 | 218 | Yes | Yes | Yes | Yes | 48 | No | Korea |
| Zhang8 | 2020 | 364 | 218 | 146 | Yes | Yes | Yes | Yes | 52 | Yes | China |
| Ryu. | 2022 | 638 | 638 | 229 | Yes | Yes | Yes | Yes | 53 | Yes | Korea |
| De Feo5 | 2022 | 229 | 161 | 161 | Yes | Yes | Yes | Yes | 57 | No | Italy |
| Meissnitzer9 | 2015 | 67 | 92 | 67 | Yes | Yes | Yes | Yes | 54 | Yes | Austria |
| Zhu10 | 2024 | 2482 | 2621 | 229 | Yes | Yes | Yes | Yes | 49 | Yes | China |
| Cho11 | 2016 | 162 | 66 | 66 | Yes | Yes | Yes | Yes | 51 | Yes | Korea |
| Zhang8 | 2020 | 390 | 229 | 229 | Yes | Yes | Yes | Yes | 56 | No | China |
Diagnostic Accuracy of Imaging Modalities
Breast-Specific Gamma Imaging (BSGI)
BSGI has demonstrated high diagnostic sensitivity and specificity in various studies. Liu4 reported a sensitivity of 91.7% and a specificity of 80.7% for BSGI in diagnosing breast lesions. Similarly, Cho11 found a sensitivity of 90.9% and a specificity of 78.1% for BSGI in their analysis of BI-RADS 4 lesions. Kim7 noted that the sensitivity of BSGI was 88.26%, with a specificity of 81.62%, indicating that BSGI is a valuable diagnostic tool, especially in dense breast tissues. In another study by Meissnitzer9, BSGI achieved a sensitivity of 90% and a specificity of 83%, further confirming its effectiveness in breast cancer detection (Figure 2).
Magnetic Resonance Imaging (MRI)
MRI has long been recognized for its superior sensitivity in detecting breast lesions. Liu4 reported a sensitivity of 92.5% and a specificity of 69.7% for MRI. In a study by Liu4, the sensitivity of MRI in detecting residual tumors following neoadjuvant chemotherapy was 83.9%, with a specificity of 58.8%. Meissnitzer9 also demonstrated that MRI had a sensitivity of 92%, though its specificity was relatively lower at 56% (Figure 2).
Mammography
Mammography remains a commonly used imaging modality for breast cancer screening, though its sensitivity can be affected by breast density. Liu1 found that mammography had a sensitivity of 77.3% and a specificity of 74.5%. Cho11 reported a slightly lower sensitivity of 74.2% and a specificity of 56.3%. In Kim7 study, mammography exhibited a sensitivity of 87.95% and a specificity of 66.83%, highlighting its limitations, particularly in dense breast tissues (Figure 2).
Ultrasound
Ultrasound is often used as an adjunct to mammography, especially in cases of dense breast tissue. In their study, Liu.1 reported a sensitivity of 82.1% and a specificity of 70.8% for ultrasound. Cho11 found that ultrasound had a sensitivity of 87.9%, though its specificity was relatively low at 19.8%. Kim7 reported a high sensitivity of 97.83% for ultrasound, but its specificity was limited to 15.2%, reflecting the higher false-positive rate associated with this modality (Figure 2).
 |
Figure 2. Forest Plot of Sensitivity and Specificity for BSGI, MRI, Mammography, and Ultrasound in Breast Lesion Diagnosis Click here to view Figure |
Positive and Negative Likelihood Ratios
Breast-Specific Gamma Imaging (BSGI)
Breast-Specific Gamma Imaging (BSGI) demonstrated a high diagnostic performance in detecting breast cancer lesions. In terms of the positive likelihood ratio (LR+), BSGI achieved a value of 4.90 (95% CI: 3.12–7.68), which indicates that patients with a positive result on BSGI were approximately five times more likely to have breast cancer compared to those with a negative result.1,7 Moreover, the negative likelihood ratio (LR-) of 0.12 (95% CI: 0.08–0.20) suggests that BSGI has a solid ability to rule out disease when the result is negative.5 These metrics confirm BSGI’s utility as a highly sensitive and specific diagnostic tool, particularly in dense breast tissues (Figure 3).
Magnetic Resonance Imaging (MRI)
Magnetic Resonance Imaging (MRI) also exhibited diagnostic solid accuracy with a PLR of 3.25 (95% CI: 2.50–4.30), showing its effectiveness in confirming disease presence.4 The NLR of MRI was 0.15 (95% CI: 0.09–0.25), indicating that MRI performs well in ruling out breast cancer when no suspicious findings are detected.6 However, while MRI’s sensitivity is excellent, its specificity is comparatively lower than BSGI (Figure 3).
Mammography
Mammography remains one of the most commonly used breast cancer screening tools. In the studies reviewed, mammography had a PLR of 2.50 (95% CI: 1.90–3.10) and an NLR of 0.22 (95% CI: 0.15–0.35).1 These values indicate that while mammography is a reliable screening tool, its diagnostic performance may be limited, particularly in dense breast tissue (Figure 3). It is often used with other imaging modalities, such as BSGI or MRI, to improve overall diagnostic accuracy.9
Ultrasound
Ultrasound is often employed as an adjunct to mammography, especially in patients with dense breast tissue. The PLR for ultrasound was 2.85 (95% CI: 2.10–3.90), demonstrating its ability to detect breast lesions.7 The NLR was 0.18 (95% CI: 0.12–0.30), suggesting that ultrasound performs reasonably well in excluding malignancy when no abnormalities are found.11 However, ultrasound is best used with other diagnostic tools like BSGI or MRI due to its operator dependency and relatively lower specificity (Figure 3).
Effect Sizes and Confidence Intervals of Diagnostic Imaging Modalities
The forest plot presents the effect sizes and confidence intervals for nine studies comparing different diagnostic imaging modalities. The effect sizes, ranging from 0.88 to 0.94, indicate the diagnostic accuracy of these modalities in detecting breast lesions. The confidence intervals for each study are relatively narrow, suggesting high precision in the estimates. Most studies show consistent performance, with effect sizes closely clustered, indicating that the diagnostic methods have comparable accuracy. Notably, the study by Zhang3 exhibited the highest effect size, while Cho11 displayed slightly lower accuracy than the others. Overall, the results highlight a reliable performance across studies, with minor variations in diagnostic efficacy (Figure 3).
 |
Figure 3: Forest Plot of Effect Sizes and Confidence Intervals for Diagnostic Imaging Studies Click here to view Figure |
The summary bivariate ROC curve illustrates the diagnostic accuracy of the included studies by plotting sensitivity against 1-specificity. The pooled sensitivity and specificity are represented by the summary operating point at 0.91 and 0.81, respectively. The SROC curve, which summarizes the overall performance of the diagnostic tests, shows a solid diagnostic capability. The 95% confidence contour (blue dashed line) and 95% prediction contour (green dotted line) reflect the variability around the summary operating point. The proximity of the observed data points to the SROC curve indicates consistent diagnostic accuracy across the studies, with relatively high sensitivity and specificity values. Overall, the results demonstrate robust diagnostic performance for the imaging modalities evaluated in this analysis (Figure 4).
 |
Figure 4: Summary Bivariate ROC Curve for Diagnostic Accuracy with 95% Confidence and Prediction Contours Click here to view Figure |
Discussion
This systematic Review critically evaluated and compared the diagnostic accuracy of Breast-Specific Gamma Imaging (BSGI), MRI, mammography, and ultrasound in the detection of breast lesions, with a focus on their correlations with specific molecular subtypes. The findings reinforce the critical role these modalities play in breast cancer diagnosis, especially in dense breast tissues. BSGI emerged as a highly sensitive and specific modality, particularly in dense breast tissues where mammography and ultrasound are often less effective.1 In agreement with Zhang and Xiao12 molecular imaging techniques, such as BSGI, provide a critical advantage by detecting metabolic activity in tumors, offering diagnostic insights beyond anatomical imaging alone.
The ability of BSGI to detect lesions in dense breast tissue was particularly notable. Dense breast tissue often reduces the sensitivity of mammography, leading to missed diagnoses.7 BSGI has demonstrated superior diagnostic performance in this context, with several studies showing a higher sensitivity and specificity than mammography and ultrasound.13 This supports the findings of Park14, who also emphasized BSGI’s ability to detect invasive breast cancer, particularly in patients with mammographically dense breasts.
MRI, another highly sensitive modality, was found to be effective in detecting breast lesions, especially in high-risk patients or those with dense breasts.1 However, MRI’s lower specificity, which can result in false positives, remains a concern.9 Studies have demonstrated that MRI often overestimates the extent of disease, leading to unnecessary biopsies and surgical interventions.4 This limitation, also noted by Huppe10, can be mitigated by combining MRI with BSGI, as the latter provides functional imaging to better differentiate between malignant and benign lesions.
Mammography remains a commonly used tool in breast cancer screening, but its limitations in dense breast tissue are well-documented.11 As Zhang8 noted, mammography’s diagnostic accuracy is compromised in dense breasts, where overlapping fibro glandular tissue can obscure lesions. The findings of this Review corroborate these concerns, highlighting the need for adjunctive imaging techniques like BSGI and MRI, particularly in patients with dense breasts.
Ultrasound, often used as a supplementary tool to mammography, showed high sensitivity but relatively low specificity.7 This is consistent with findings from previous studies, such as those by Viviani15, who noted that ultrasound is prone to false positives, leading to unnecessary biopsies. While ultrasound helps characterize lesions, particularly cystic ones, its operator-dependent nature can result in variability in diagnostic accuracy.16 This variability underscores the need for supplementary imaging techniques such as BSGI, which relies less on operator expertise.
One of the key findings of this Review is the growing evidence supporting the use of BSGI as a complementary imaging modality in breast cancer diagnostics. Studies by Ryu6 and De Feo5 have demonstrated the added value of BSGI in reducing false positives, particularly in patients with dense breast tissue. BSGI’s ability to detect metabolic activity in tumors provides a functional perspective that complements the anatomical imaging provided by mammography and ultrasound, improving diagnostic accuracy.
Furthermore, BSGI has shown promise in correlating imaging findings with specific molecular subtypes of breast cancer. HER2-positive and triple-negative breast cancers, which tend to exhibit higher metabolic activity, are more likely to be detected using BSGI.4 This is particularly important in personalized medicine, where accurate identification of tumor subtypes can guide treatment strategies. This finding aligns with research by Viviani15 who noted that molecular imaging techniques like BSGI can predict neoplasms based on background uptake of fibroglandular tissue.
A significant strength of BSGI is its utility in patients with inconclusive findings from traditional imaging modalities. Ko2 emphasized BSGI’s cost-effectiveness in avoiding unnecessary biopsies in patients with indeterminate mammography or ultrasound results. This Review supports the notion that BSGI, when used as a problem-solving tool, reduces the need for follow-up interventions, leading to more efficient diagnostic pathways and improved patient outcomes.17
In contrast, one limitation noted in MRI and ultrasound is the potential for false positives, which can lead to overdiagnosis and overtreatment.3 The lower specificity of these modalities can result in unnecessary biopsies and surgeries, particularly in high-risk patients or those with dense breasts. Studies by Zhan and Sun13 highlighted the importance of using BSGI as an adjunct to MRI to improve diagnostic specificity and reduce false-positive results, particularly in challenging cases involving dense breast tissue or high-risk patients.
While the findings of this systematic review emphasize the superior diagnostic accuracy of Breast-Specific Gamma Imaging (BSGI) compared to mammography, MRI, and ultrasound, alternative hypotheses and interpretations of the data merit consideration. For instance, the variations in specificity and sensitivity across studies could be influenced by differences in patient demographics, imaging protocols, and operator expertise. The higher specificity of BSGI, particularly in dense breast tissues, suggests its potential as a complementary tool. However, the differences in cost, accessibility, and radiation exposure associated with BSGI compared to the other modalities warrant further investigation, especially in resource-limited settings where cost-effectiveness plays a crucial role in clinical decision-making. These factors might limit its widespread adoption despite its demonstrated diagnostic advantages.
Additionally, the observed correlations between imaging findings and molecular subtypes of breast cancer, such as HER2-positive and triple-negative cancers, open avenues for more targeted diagnostic strategies. However, alternative explanations for these findings could include inherent variability in tumor metabolism or imaging parameters rather than a direct superiority of BSGI. Future research could explore integrating imaging modalities with emerging technologies such as artificial intelligence to refine the diagnostic accuracy further. Additionally, longitudinal studies assessing patient outcomes related to imaging findings would provide more robust evidence for the clinical utility of combining these imaging techniques. Addressing these gaps will strengthen the applicability of these findings and inform their integration into clinical workflows.
Finally, the findings of this systematic Review highlight the importance of integrating multiple imaging modalities in breast cancer diagnostics. While mammography and ultrasound remain essential tools, advanced imaging techniques such as BSGI and MRI offer significant advantages, particularly in patients with dense breast tissue or inconclusive findings from traditional imaging methods. As imaging technology continues to evolve, the role of BSGI in breast cancer diagnostics is expected to expand, offering clinicians a valuable tool for improving diagnostic accuracy and patient outcomes.10
Conclusion
In conclusion, this systematic Review highlights the comparative strengths and limitations of BSGI, MRI, mammography, and ultrasound in diagnosing breast lesions, particularly in dense breast tissues and their correlation with molecular subtypes. BSGI emerged as a valuable adjunct imaging modality with high sensitivity and specificity, especially in cases where mammography and ultrasound alone may fall short. While highly sensitive, MRI often produces false positives, leading to overdiagnosis. Combining BSGI with imaging modalities like MRI can enhance diagnostic accuracy, reducing unnecessary biopsies. Overall, integrating these advanced imaging tools improves breast cancer diagnostics, allowing for more personalized and precise treatment strategies and ultimately enhancing patient outcomes.
Implications and Recommendations
The findings of this systematic review underscore the importance of integrating advanced imaging modalities, such as BSGI and MRI, into clinical workflows for breast cancer diagnostics. The ability of BSGI to detect metabolically active lesions, particularly in dense breast tissues, offers a significant advantage over traditional modalities like mammography and ultrasound. This makes it a critical tool in reducing diagnostic uncertainty and enhancing the early detection of breast lesions, which is crucial for improving treatment outcomes. The observed correlations between imaging findings and molecular subtypes further highlight the potential of these modalities to support personalized medicine by tailoring treatment strategies based on tumor characteristics. These insights could transform current diagnostic practices, providing a pathway to more accurate and individualized care for breast cancer patients.
To translate these findings into clinical practice, several recommendations can be made. Healthcare providers should consider incorporating BSGI as a complementary imaging technique, particularly in cases of dense breast tissue or inconclusive findings from mammography and ultrasound. Training programs should be developed to familiarize radiologists and clinicians with the capabilities and limitations of BSGI to maximize its clinical utility. Additionally, future research should focus on cost-effectiveness analyses to evaluate the feasibility of widespread implementation and explore how BSGI can be integrated with other diagnostic advancements, such as artificial intelligence. Finally, policymakers and healthcare institutions should prioritize access to advanced imaging modalities to bridge the gap in diagnostic accuracy and promote equitable healthcare delivery. These steps will not only improve diagnostic precision but also contribute to better patient outcomes and resource optimization in breast cancer care.
Acknowledgment
We would like to extend our gratitude to all contributors who supported this study, particularly our colleagues from the Department of Breast Imaging and Diagnostic Radiology. We appreciate the invaluable insights and assistance provided throughout the research process. Special thanks to the institutions and teams involved for their collaboration and commitment. We are also grateful to our peers and mentors for their constructive feedback, which helped enhance the quality of this systematic review and meta-analysis.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The author(s) do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration- This research does not involve any clinical trials
Author Contributions
- Reem Kalakattawi: Conceptualization, Methodology, Writing – Original Draft.
- Lena Gowharji: Data Collection, Analysis, Writing – Review & Editing.
- Alaa Marzogi: Visualization, Supervision, Project Administration.
- Tahani Alghamdi: Resources, Supervision.
All authors have significantly and directly contributed intellectually to the project and have approved the final version of the manuscript for publication.
References
- Liu H, Zhan H, Sun D, Zhang Y. Comparison of BSGI, MRI, mammography, and ultrasound for the diagnosis of breast lesions and their correlations with specific molecular subtypes in Chinese women. BMC Medical Imaging. 2020;20:1-10.
CrossRef - Ko A, Vo AM, Miller N,. The Use of Breast-specific Gamma Imaging as a Low-Cost Problem-Solving Strategy for Avoiding Biopsies in Patients With Inconclusive Imaging Findings on Mammography and Ultrasonography. Journal of Breast Imaging. 2024;6(5):502-512.
CrossRef - Zhang Y, Zhu D, Feng R. Breast-specific gamma imaging versus ultrasound and mammography for breast cancer diagnosis: A meta-analysis. International Journal of Radiation Research. 2024;22(1):27-33.
CrossRef - Liu H, Zhan H, Zhang Y,. Comparison of BSGI and MRI as Approaches to Evaluating Residual Tumor Status after Neoadjuvant Chemotherapy in Chinese Women with Breast Cancer. Diagnostics. 2021;11(10):1846.
CrossRef - De Feo MS, Sidrak MMA, Conte M,. Breast-Specific gamma imaging: An added value in the diagnosis of breast cancer, a systematic review. Cancers. 2022;14(19):4619.
CrossRef - Ryu RR, Kim YJ, Seo JY, Kim KW, Kim JS. Breast-specific Gamma Imaging (BSGI) as a Complementary Imaging Tool for BI-RADS 0 and 4a Lesions on Mammography or Ultrasonography. Iranian Journal of Radiology. 2022;19(2)
CrossRef - Kim YJ, Seo JY, Kim KW, Hwang CM, Oh DH. The usefulness of addition of breast-specific gamma imaging to mammography in women with dense breast. Egyptian Journal of Radiology and Nuclear Medicine. 2023;54(1):94.
CrossRef - Zhang Z, Wang W, Wang X,. Breast-specific gamma imaging or ultrasonography as adjunct imaging diagnostics in women with mammographically dense breasts. European Radiology. 2020;30:6062-6071.
CrossRef - Meissnitzer T, Seymer A, Keinrath P,. Added value of semi-quantitative breast-specific gamma imaging in the work-up of suspicious breast lesions compared to mammography, ultrasound and 3-T MRI. The British journal of radiology. 2015;88(1051):20150147.
CrossRef - Huppe AI, Mehta AK, Brem RF. Molecular breast imaging: a comprehensive review. Elsevier; 2018:60-69.
CrossRef - Cho MJ, Yang J-H, Yu YB,. Validity of breast-specific gamma imaging for Breast Imaging Reporting and Data System 4 lesions on mammography and/or ultrasound. Annals of Surgical Treatment and Research. 2016;90(4):194.
CrossRef - Zhang X-H, Xiao C. Diagnostic value of nineteen different imaging methods for patients with breast cancer: a network meta-analysis. S. Karger AG Basel, Switzerland; 2018. p. 2041-2055.
CrossRef - Zhan H, Sun D. Comparison of 99mTc-MIBI scintigraphy, ultrasound, and mammography for the diagnosis of BI-RADS 4 category lesions. 2020;
- Park JY, Chun KA, Shin HJ, Yi SY, Kwon H-J, Park HJ. Breast-specific gamma imaging of invasive breast cancer: Clinicopathologic factors affecting detectability and correlation with mammographic findings. Clinical Imaging. 2018;51:168-173.
CrossRef - Viviani CLS, Veras LP, Viviani DN, de Amorim RFB. Molecular breast imaging and background uptake of fibroglandular tissue as tools to predict neoplasms in dense breasts. Mastology. 2021;31:1-9.
CrossRef - Brem RF, Mehta AK, Rapelyea JA, Akin EA, Bazoberry AM, Velasco CD. Gamma Imaging–Guided Minimally Invasive Breast Biopsy: Initial Clinical Experience. American Journal of Roentgenology. 2018;210(3):695-699.
CrossRef - Yu X, Dong M, Yang D, Wang L, Wang H, Ma L. Deep learning for differentiating benign from malignant tumors on breast-specific gamma image. Technology and Health Care. 2023;31(S1):61-67.
CrossRef
Abbreviations
BSGI – Breast-Specific Gamma Imaging, MRI – Magnetic Resonance Imaging, PRISMA – Preferred Reporting Items for Systematic Reviews and Meta-Analyses, QUADAS-2 – Quality Assessment of Diagnostic Accuracy Studies-2, DOR – Diagnostic Odds Ratio, SROC – Summary Receiver Operating Characteristic, AUC – Area Under the Curve, HER2 – Human Epidermal Growth Factor Receptor 2, TN – True Negative, TP – True Positive, FN – False Negative, FP – False Positive, LR+ – Positive Likelihood Ratio, LR- – Negative Likelihood Ratio, CI – Confidence Interval, STATA – Statistical Software.







