Sandhya Parameswaran * and Rohan Rajkumar
and Rohan Rajkumar
Department of Quality Assurance, Saraswathi Vidya Bhavan`s college of Pharmacy, Dombivli, Maharashtra, India
Corresponding Author E-mail:Sandhya.parmeswaran@svbpharmacy.edu.in
DOI : https://dx.doi.org/10.13005/bpj/3120
Abstract
Nutritional therapy and phytotherapy have surfaced as new theories in healing systems, with a strong recommendation for utilising nutraceuticals and natural plant foods as stand-alone treatments or combined with allopathic medicine. This research paper presents the formulation and evaluation of a bilayer tablet comprising beetroot and isoniazid, which is a drug of choice for tuberculosis (TB). TB increases circulating hepcidin levels, impairing dietary iron absorption and systemic utilization, often leading to anaemia. Beetroot (Beta vulgaris, Family Chenopodiaceae), known for being rich in carbohydrates, folate, minerals, and vitamin C, was chosen for its potential to address this issue. Linearity curves established by UV spectroscopy showed r² values of 0.992 for isoniazid (concentration 6-16 ppm) and r²=0. 998 for iron (0.1-2 ppm). The beetroot extract was spray-dried, and on analysis, its iron content was 0.115% w/w. A 500 mg bilayer tablet was formulated, containing 100 mg of isoniazid and 250 mg of beetroot powder, equivalent to 0.287 mg of iron, using sodium starch glycolate and croscarmellose sodium as disintegrants. The tablet was evaluated based on parameters like hardness, friability, drug content, disintegration, and dissolution. The optimal formulation, which included equal amounts of both disintegrants, demonstrated a drug content of 101-102% for both isoniazid and iron, a disintegration time of 190 seconds, and a 100% release rate at the end of 45 minutes.
Keywords
Anaemia; Beetroot; Bilayer tablet; Isoniazid; Tuberculosis
Download this article as:| Copy the following to cite this article: Parameswaran S, Rajkumar R. Bilayer Tablet Formulation of Beetroot and Isoniazid: Integration of Herbal Nutrition and Anti-Tubercular Therapy. Biomed Pharmacol J 2025;18(1). |
| Copy the following to cite this URL: Parameswaran S, Rajkumar R. Bilayer Tablet Formulation of Beetroot and Isoniazid: Integration of Herbal Nutrition and Anti-Tubercular Therapy. Biomed Pharmacol J 2025;18(1). Available from: https://bit.ly/3EECWnG |
Introduction
Plants are among the most valuable resources for human food and medicine. Nutritional therapy and phytotherapy have surfaced as prominent therapeutic systems, gaining rapid and widespread adoption in recent years. There is now a strong recommendation for the consumption of nutraceuticals and natural plant foods, either as a single drug or in combination with allopathic medicine1. However, herb-food and herb-drug interactions should be carefully studied before initiating any combination therapy. The use of nutritional therapy derived from herbs is becoming increasingly popular, with a growing trend towards using these nutrient-rich herbs as complementary therapies alongside synthetic drugs. This inclination towards herbal nutrients has paved the way for studying the dietary nutritional values of fruits and vegetables, which have recently become targets for enhancing immunity.
Beetroot (Beta vulgaris), belonging to the Chenopodiaceae family, often denoted by the phrase “red blood.” is a potent source of various phytochemical compounds, comprising ascorbic acid, carotenoids, phenolic acids, and flavonoids. Beetroot’s nutritional value is high due to its significant content of carbohydrates, folate, fibre, and minerals such as iron, nitrate, manganese, potassium, and vitamin C 2. Additionally, beetroot’s attributes include being fat-free, low in calories, and cost-effective. Traditional medicine suggests using red beet juice as a source of iron for treating anaemia, although literature indicates that the aspect of iron absorption from beetroot is the least studied. The high levels of minerals and nutrients in beetroot also support its use in antidepressant, antimicrobial, and anti-carcinogenic therapies alongside main drug treatments. Nutritional values per 100 grams of beetroot include 8 mg of iron, 136.0 mcg of folic acid, 1.9 g of potassium, 6.12 mg of vitamin C, and 39.1 g of magnesium2.
Tuberculosis (TB) is a chronic granulomatous infection and remains a significant health concern in developing countries. Anaemia is a well-known complication of TB, with two primary causes: anaemia due to inflammation, which is primarily a result of TB itself and does not respond to iron therapy, and iron-deficiency anaemia, which does respond to iron therapy. TB increases circulating hepcidin levels, impairing dietary iron absorption and systemic iron utilisation, leading to iron sequestration and anaemia3,4. Consequently, TB patients often exhibit decreased haemoglobin levels, which can directly impact TB-associated morbidity5. The primary drug used to treat all forms of tuberculosis is isoniazid, also known as isonicotinic acid hydrazide (INH) or pyridine-4-carboxylic acid hydrazide. INH is a pro-drug that inhibits InhA, an enzyme critical for mycolic acid biosynthesis, a key component of the mycobacterial cell wall, leading to the destruction of the bacterial cell and ultimately causing cell death. INH is quickly and almost completely absorbed (90-95%) from the gastrointestinal tract and is available in combination with pyridoxine, rifampicin, pyrazinamide, streptomycin or ethambutol. Given its essential role in medical treatment, accurately determining INH in pharmaceutical formulations and biological fluids is crucial for public health programs. 6.
This project builds upon previous research conducted at our institute, which focused on the study of the bioavailability of iron from beetroot as compared to marketed iron preparations. That study, conducted on Albino Wistar rats, measured blood parameters such as RBC, WBC, and haemoglobin over a 30-day period and gave positive results7. The present research extends this work by developing a combination formulation for use in tuberculosis treatment. Given that anaemia is a common complication of TB, we aimed to create a formulation containing isoniazid, the most commonly used anti-TB drug, combined with beetroot extract.
Materials and Methods
Materials
Beta vulgaris root was purchased from a general market and authenticated by Dr. Harshad M. Pandit from Guru Nanak Khalsa College, Mumbai, Maharashtra, India. Isoniazid was procured from M/s Yarrow Chem Pvt Ltd.
Methods
Preparation and Evaluation of Beetroot Extract
Beetroot extract was prepared by autoclaving the required amount of crude drugs (50% beetroot, 10% ginger, and 5% w/v amla powder) in 75 ml of water at 121°C for 15 minutes under 15-pound pressure8. After autoclaving, the extract was cooled and filtered through muslin cloth. Lemon juice (5% w/v) was added, and the volume was brought up to 100 ml. The extract was studied for organoleptic properties and iron content using UV-Vis spectroscopy.
Standard Curve for Iron
A standard ferrous solution (10 ppm) was obtained by dissolving 0.0702 g of ferrous ammonium sulphate in 500 ml of water. To this solution, 2.5 ml of concentrated sulfuric acid was added, and the volume was brought up to 1000 ml with water. For the standard curve, different volumes were pipetted from the 10-ppm solution into a 100 ml capacity volumetric flask to obtain concentrations of 0.1, 0.2, 0.3, 0.5, 1.0, 1.5, and 2.0 ppm. To each flask, 1 ml of hydroxylamine hydrochloride and 5 ml of 1,10-phenanthroline were added, and the solutions were buffered with 8 ml of acetic acid/sodium acetate buffer9. The solutions were kept in the dark for 15 minutes to allow full colour development. After 15 minutes, the solutions were made up to the 100 ml mark, and the absorbance of each solution was measured at λ max 510nm using an ELICO UV SL159 Spectrophotometer against a blank.
Analysis of Beetroot Extract
A 10 ml sample of beetroot extract was placed in a 100 ml volumetric flask. This was followed by the addition of 1 ml of hydroxylamine hydrochloride and 5 ml of 1,10-phenanthroline. This solution was buffered using 8 ml of acetic acid/sodium acetate buffer. The sample was kept in the dark for 15 minutes and then made up to the 100 ml mark with water. The absorbance was recorded at λ max 510 nm.
Spray Drying of Beetroot Extract
The beetroot extract was spray dried using an LSD-48 MINI SPRAY DRYER. The operational parameters for spray drying included an inlet air temperature of 120°C – 150°C, an airflow rate of 118 Nm³/hr, and a feed flow rate of 150 ml/hr of water. Maltodextrin, at a concentration of 5% w/v, was added to the extract as a drying agent10.
Analysis of Iron Content in the Spray-Dried Extract
To analyse the iron content, 10 mg of spray-dried extract was dissolved in water, and the volume was brought up to 10 ml in a volumetric flask. From this solution, 10 ml was pipetted and added to a 100 ml volumetric flask, thereafter solutions hydroxylamine hydrochloride (1ml) and 1,10-phenanthroline (5ml) were added. Acetic acid/sodium acetate buffer (8ml) was added to buffer the above solution. A dark time of 15 minutes was maintained and then volume was brought up to 100 ml mark with water. The absorbance was recorded at λ max 510 nm against a blank.
Standard Curve of Isoniazid
A 1000ppm solution of Isoniazid was obtained by dissolving 10 mg of accurately weighed drug in water and volume brought up to 10ml in a volumetric flask. A standard curve for isoniazid was prepared with concentrations of 6, 8, 10, 12, 14, and 16 ppm. The standard curve was measured against a reagent blank containing beetroot extract. This was done to nullify the absorbance of beetroot extract which may interfere during the assay of the formulation as it contained both beetroot and isoniazid10.
Compatibility Studies of the Beetroot powder with isoniazid
A mixture of 300 mg of isoniazid and 700 mg of spray-dried beetroot powder was studied for 30 days at room temperature to evaluate its compatibility11. For the determination of isoniazid content, 10mg of the sample was dissolved in 10 ml of water and further diluted 100 times. The absorbance was recorded at λ max 263 nm, in UV Spectrophotometer in intervals of 0,3, 7,15 & 30 days. For iron content determination, 10mg of the sample was dissolved in 10ml of water and analysed by the 1,10 phenanthroline method at λ max 510 nm on the same interval days.
Formulation of Bilayer Tablet
A bilayer tablet containing beetroot extract and isoniazid was prepared as per the formula stated in Table No.1, using a 12 mm punch on a single punch machine. The flow properties of the granules were assessed by inferring the results of various parameters like angle of repose, bulk density, tapped density, compressibility index (CI), and Hausner’s ratio (HR). The compressed tablets were assessed based on their physical appearance, weight variation, hardness (using a Monsanto hardness tester), friability (using a Roche friability tester), drug content, disintegration time (using a disintegration test apparatus), and dissolution (using a USP dissolution test apparatus type 2 Paddle)12,13.
Table 1: Formulae for bi layered tablet
| Ingredient | F1 | F2 | F3 | |||
| Per Tablet | Isoniazid | Dried beetroot | Isoniazid | Dried beetroot | Isoniazid | Dried beetroot |
| Drug | 100mg | 250mg | 100mg | 250mg | 100mg | 250mg |
| Avicel | 35mg | 65mg | 35mg | 65mg | 35mg | 65mg |
| Sodium starch glycolate | 11mg | 26mg | – | – | 5.5mg | 13mg |
| Croscarmellose sodium | – | – | 11mg | 26mg | 5.5mg | 13mg |
| Magnesium stearate | 3mg | 6mg | 3mg | 6mg | 3mg | 6mg |
| Talc | 1mg | 3mg | 1mg | 3mg | 1mg | 3mg |
| IPA | q.s | q.s | q.s | q.s | q.s | q.s |
Results and Discussion
Preparation and evaluation of beetroot extract
The crude drug Beta vulgaris (Beetroot) was authenticated by Dr. Harshad M. Pandit, Head and Associate Professor of Botany, and assigned voucher number: PPP P 04214917. The beetroot was characterized by a deep red colour, earthy odour, sweet taste, a moisture content of 5.8% w/w, and a total ash value of 5.1% w/w. The beetroot extract had an extractive value of 8.01 ± 0.09% w/w.
Standard curve of iron and isoniazid
The linearity curve of iron (Fig. 1) was established at a range of 0.1-2.0 ppm, and showed a correlation coefficient (r²) of 0.998 and a standard equation of Y = 0.2106x + 0.0015. The prepared beetroot extract was analysed for iron content using this method and contained 0.132% w/w of iron. The standard curve for Isoniazid (INH) was linear in the concentrations ranging from 6 ppm to 16 ppm at λ max 263 nm (Fig.2). Linearity was established with a correlation coefficient (R²) of 0.992 with a standard curve equation determined at Y = 0.0695x – 0.2732.
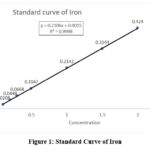 |
Figure 1: Standard Curve of IronClick here to view Figure |
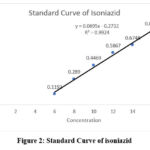 |
Figure 2: Standard Curve of isoniazid Click here to view Figure |
Evaluation of spray-dried powder of beetroot extract
The beetroot extract was spray-dried using 5% maltodextrin. The resulting spray-dried powder was pink in colour, with a sweet taste, and was evaluated for physical properties, including bulk density (0.55 ± 0.007 g/ml), tapped density (0.63 ± 0.014 g/ml), moisture content (3.6 ± 0.25%), and compressibility index (12.69%). The spray-dried powder was also analysed for iron content and gave a value of 0.115 ± 0.004% w/w.
Compatibility Studies
The content of iron and INH in the test samples used for compatibility studies was calculated using the respective standard curve equations. The content of iron and INH on day zero was 69.8 mg% w/w and 95.93% w/w respectively and that on the 30th day was 65.5 mg% w/w and 98.57% w/w respectively. The relative standard deviation for iron and INH content measured at intervals of 0,3,7,15 & 30 days was 2.57 and 1.26 respectively. These results given in Table No.2 indicated a change of only ±5% in the content of iron and INH during the one-month study period, suggesting good compatibility between the components.
Table 2: Results for compatibility studies of beetroot spray dried powder and INH
| Day | IRON (%w/w) | INH (% w/v) |
| Day 0 | 69.8 | 95.93 |
| Day 3 | 68.8 | 95.4 |
| Day 7 | 67.9 | 96.69 |
| Day 15 | 66.4 | 97.16 |
| Day 30 | 65.5 | 98.57 |
| Standard deviation | ±1.74 | ±1.22 |
| RSD | 2.57 | 1.26 |
Formulation of Solid Dosage Form: Pre-Compression Parameters of the Granules Ready for Compression (GRC) Blends
Two sets of granules were prepared: one for the GRC of INH and the other for the GRC of spray-dried beet root extract (DBR), as per the formulation provided in Table No. 1. Three different formulations were prepared, each with varying concentrations of disintegrants, namely Sodium Starch Glycolate and Croscarmellose Sodium. The results of the pre-compression parameters related to the flow properties of the granules were evaluated and recorded in Table 3. It was observed that the formulations F1 and F2 had good flow properties while F3 had excellent flow. All three formulations showed a fair compressibility Index in the increasing order of F3 better than F2, then F1 (F3>F2>F1)
Table 3: Results for precompression parameters of the GRC blends
| Formulations | Angle of repose (°) | Bulk density (g/ml) | Tapped density(g/ml) | Compressibility index (%) | Hausner’s ratio | |||||
| INH | DBR | INH | DBR | INH | DBR | INH | DBR | INH | DBR | |
| F1 | 33.02 | 32.25 | 0.47 | 0.45 | 0.60 | 0.59 | 21.60 | 23.33 | 1.27 | 1.31 |
| F2 | 30.11 | 30.07 | 0.51 | 0.54 | 0.63 | 0.67 | 19.06 | 19.40 | 1.23 | 1.24 |
| F3 | 29.24 | 28.75 | 0.50 | 0.45 | 0.60 | 0.56 | 16.60 | 19.6 | 1.20 | 1.24 |
Post-compression evaluation results of the prepared tablets
A bilayer tablet was prepared as per the formulae of F3 formulation, containing 100 mg of Isoniazid (INH) and 250 mg of spray-dried beetroot powder Fig. 3. The tablets were compressed using a 12 mm die on a single-punch tablet machine, with each tablet weighing approximately 500 mg. The tablets underwent evaluation for key quality parameters, including weight variation, hardness, friability, disintegration time, and in vitro dissolution performance using a USP Type II dissolution test apparatus. The results are given in Table 4
The drug content was calculated based on the concentration of active ingredients in each tablet: 100 mg of INH and 250 mg of spray-dried beetroot powder, equivalent to 0.287 mg of iron (based on an iron content of 0.115% w/w in the spray-dried powder). In vitro dissolution studies were conducted in 900 mL of 0.1 N HCl at 37 ± 0.5°C with a paddle speed of 100 rpm. The results as given in Fig 4 indicated a 100% cumulative release of the active drug within 1 hour, demonstrating effective dissolution characteristics of the bilayer tablet formulation.
 |
Figure 3: Bilayered Tablet of beetroot extract (spray dried) and Isoniazid Click here to view Figure |
Table 4: Results for post compression parameters of bilayered tablets.
| Formulations | Weight variation (mg) | Hardness (kg/cm2) |
Friability | Disintegration time (min) | INH (%) w/w | Beetroot (%) w/w |
| F1 | 501.10 ±0.063 | 2.5±0.07 | 1.6±0.002 | 4.31±0.09 | 105.30±0.34 | 99.1±0.05 |
| F2 | 499.75 ±0.025 | 3.0±0.02 | 1.3±0.003 | 5.45±0.22 | 101.03±0.06 | 104.0±0.007 |
| F3 | 500.25 ±0.058 | 2.5±0.07 | 1.0±0.07 | 3.10±0.10 | 102.02±0.04 | 101.6±0.03 |
 |
Figure 4: Graph depicting the invitro dissolution of bilayered tablet.Click here to view Figure |
Conclusion
Based on the compatibility results of both beetroot extract and Isoniazid (INH), a bilayer tablet weighing 500 mg was successfully formulated. Sodium starch glycolate and Croscarmellose sodium were used as disintegrants in varying ratios.
Upon evaluating the pre-compression parameters of the granule blend, it was observed that formulation F3, which contained a combination of both disintegrants, exhibited excellent flow properties, showed superior performance with consistent drug content and achieved complete drug release within one hour. This indicated that F3 is an optimal formulation for ensuring rapid and efficient release of both the active drug and the nutritional component, making it a promising candidate for addressing the dual therapeutic needs in tuberculosis management, particularly in patients with concurrent anaemia.
Acknowledgement
The authors are thankful to SVB College of Pharmacy for providing the necessary laboratory for the completion of the research work
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The author(s) do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials
Author Contributions
- Sandhya Parameswaran : Project Supervision, Data Analysis, writing, Review and editing
- Rohan Rajkumar : Project conduction, methodology , Writing- original draft
References:
- Zhao J. Nutraceuticals, nutritional therapy, phytonutrients, and phytotherapy for improvement of human health: a perspective on plant biotechnology application. Recent patents on biotechnology, 2007; 1(1):75-97. 2174/187220807779813893
CrossRef - Clifford T, Howatson G, West DJ, Stevenson EJ. The potential benefits of red beetroot supplementation in health and disease. Nutrients. 2015 ; 7(4):2801-22. 3390/nu7042801
CrossRef - Dasaradhan T, Koneti J, Kalluru R, Gadde S, Cherukuri SP, Chikatimalla R. Tuberculosis-Associated Anaemia: A Narrative Review. Cureus. 2022 ;14(8):e27746. 7759/cureus.27746
CrossRef - Nienaber A, Uyoga MA, Dolman-Macleod RC, Malan L. Iron status and supplementation during tuberculosis. Microorganisms. 2023 ;11(3):785. 3390/microorganisms11030785
CrossRef - Lee SW, Kang YA, Yoon YS, Um SW, Lee SM, Yoo CG, Kim YW, Han SK, Shim YS, Yim JJ. The Prevalence and Evolution of Anaemia Associated with Tuberculosis. J Korean Med Sci. 2006 ;21(6):1028-1032. 3346/jkms.2006.21.6.1028
CrossRef - Silva AM, Abrahim-Vieira B, do Carmo FA, do Amaral LH, Silva LC, Escudini CS, Lopes MA, Sousa VP, Castro HC, Veiga F, Rodrigues CR. Segregated delivery of rifampicin and isoniazid from fixed dose combination bilayer tablets for the treatment of tuberculosis. British Journal of Pharmaceutical Research. 2014;4(14):1781-801.
CrossRef - Patil, P.; Utilization of herbal nutrients in pharmaceutical dosage form, (M. Pharm dissertation) University of Mumbai, June, 2021.
- Atul D, Rajat S, Divya SC, Dhruv T, Shubham C, Pramod KP. Status of beetroot processing and processed products: Thermal and emerging technologies intervention.Trends in Food Science & Technology. 2021;114: 443-458. https://doi.org/10.1016/j.tifs.2021.05.042.
CrossRef - Harvey Jr AE, Smart JA, Amis ES. Simultaneous spectrophotometric determination of iron (II) and total iron with 1, 10-phenanthroline. Analytical Chemistry. 1955 ;27 (1): 26-29. https://doi.org/10.1021/ac60097a009
CrossRef - Jain P. Studies on production process optimization of beet root powder using spray dryer and its comparison with conventional process (Doctoral dissertation, JNKVV, 2013).
- Narang AS, Rao VM, Raghavan KS. Excipient compatibility. In. developing solid oral dosage forms. Academic Press. 2009: 125-145. https://doi.org/10.1016/B978-0-444-53242-8.00006-0
CrossRef - Kumar V, Upadhyay P & Kesri K. Formulation and evaluation of sustained release matrix tablets of isoniazid, World Journal of Pharmacy and Pharmaceutical Sciences, 2019; 8(5):1035-1061. 20959/wjpps20195-13722
- Mwangi MW, Tirop LJ, Njogu PM, Bururia JM, Njuguna NM, Mbae EG. Formulation of dispersible isoniazid/pyridoxine fixed-dose combination tablets for isoniazid preventive therapy in paediatrics. Cogent Medicine. 2020 ;7(1): 1787694. https://doi.org/10.1080/2331205X.2020.1787694
CrossRef







