Rajappan Chandra Satish Kumar1,2, Chittaranjan Das1* , Mothishwaran Bhuvaneshwaran2
, Mothishwaran Bhuvaneshwaran2 and Kunka Mohanram Ramkumar3
and Kunka Mohanram Ramkumar3
1Sri Jayendra Saraswathi Ayurveda College and Hospital, Sri Chandrashekarendra Viswa Vidyalaya, Kanchipuram, Tamil Nadu, India.
2Clinical Trial and Research Unit, Interdisciplinary Institute of Indian System of Medicine, SRM Institute of Science and Technology, Kattankulathur, Tamil Nadu, India.
3Department of Biotechnology, School of Bioengineering, SRM Institute of Science and Technology, Kattankulathur, Tamil Nadu, India
Corresponding Author E-mail: drcrdasayush@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/3112
Abstract
Diabetes mellitus is a chronic metabolic disorder associated with high rates of morbidity and mortality globally. Ayurvedic medicine offers promising approaches for managing diabetes, with Niruryadi Gulika (NG) emerging as a potential therapeutic agent. This study evaluated the effects of NG in comparison to metformin, a widely used antidiabetic drug, in in streptozotocin (STZ)-induced diabetic rats. Experimental groups included control, diabetic control, metformin-treated and NG-treated groups, monitored over a 6-week period. Various parameters included circulating glucose levels, plasma insulin, total haemoglobin, glycosylated haemoglobin, lipid profile (cholesterol, triglyceride, HDL, LDL), antioxidant enzymes (catalase, GPx), and lipid peroxidation (TBARS) were assessed. Our results demonstrated that NG particularly at two doses, significantly reduced blood glucose levels, glycosylated haemoglobin, and lipid peroxidation, while enhancing plasma insulin levels, total haemoglobin, and antioxidant enzyme activity compared to diabetic control rats. NG also improved lipid profile markers, such as cholesterol and triglycerides, achieving effects comparable to metformin. Furthermore, NG treatment reduced elevated cytokine levels, including TNF-α and IL-6, in diabetic rats, reflecting the anti-inflammatory effects of metformin. These findings highlight NG’s potential as an effective anti-diabetic agent, showing promising results comparable to metformin. Further research is warranted to better understand the mechanisms of NG and optimize its dosage regimen for managing diabetes mellitus.
Keywords
Antioxidants; Ayurvedic drugs; Diabetes; Niruryadi Gulika; Oxidative stress
Download this article as:| Copy the following to cite this article: Kumar R. C. S, Das C, Bhuvaneshwaran M, Ramkumar K. M. Antidiabetic Activity of Ayurvedic Niruryadi Gulika in Experimental Diabetes. Biomed Pharmacol J 2025;18(1). |
| Copy the following to cite this URL: Kumar R. C. S, Das C, Bhuvaneshwaran M, Ramkumar K. M. Antidiabetic Activity of Ayurvedic Niruryadi Gulika in Experimental Diabetes. Biomed Pharmacol J 2025;18(1). Available from: https://bit.ly/42vTYye |
Introduction
“Inducing diabetes in animal models has provided valuable insights into the physiological and biological disturbances connected with this metabolic disorder1. Notably, hyperglycemic animals have been extensively studied, revealing significant alterations in lipid metabolism. Diabetes Mellitus is a complex condition characterized by high blood sugar levels (hyperglycemia) due to issues with the production and use of insulin in the body. Diabetes is classified into two main categories: Type 1 Diabetes mellitus, which is caused by insufficient insulin production, and Type 2 Diabetes Mellitus, which is caused by the occurrence of insulin resistance in the cells and eventual buildup of extraneous insulin in the body 2. In diabetes, pathological changes are primarily related to redox imbalance and are closely associated with the onset of cardiovascular complications 3. Studies in diabetic rats have shown increased lipid peroxidation, particularly in association with hyperlipidemia 4. The liver, a crucial insulin-dependent organ responsible for maintaining glucose and lipid homeostasis, undergoes significant dysfunction during diabetes 5. The liver’s functions, including the absorption, oxidation, and energy-based transformation of non-esterified fatty acids, the production of triglycerides, cholesterol, phospholipids are significantly impaired in diabetes. This disruption often results in marked alterations in lipid concentration and composition 6. Moreover, diabetes adversely affects glucose metabolism in the liver, leading to reduced glycolysis, impaired glycogenesis, and increased gluconeogenesis 7.
In recent years, the use of complementary medicine has seen significant growth, particularly in dietary interventions and traditional plant-based therapies derived from systems such as Ayurveda. Drawing upon centuries of accumulated knowledge and experience, Ayurveda emphasizes personalized treatment plans based on an individual’s constitutions, or doshas, comprising Vata, Pitta, and Kapha. Ayurvedic therapies for diabetes encompass a holistic and multifaceted approach, including dietary modifications, herbal remedies, lifestyle changes, and specialized treatments like Panchakarma detoxification 8.
Key herbs commonly used in Ayurveda for diabetes management are, bitter gourd (Momordica charantia) and fenugreek (Trigonella foenum-graecum), and Indian gooseberry (Emblica officinalis), all known for their potent blood sugar-regulating properties 9-11. Ayurvedic principles underline the importance of maintaining balance in bodily functions while optimizing digestion, metabolism, and elimination to efficiently prevent and manage diabetes effectively. By addressing the root causes of diabetes and promoting overall well-being, Ayurvedic medicine offers a comprehensive and integrative approach to diabetes care. This holistic framework complements conventional treatments, offering patients a pathway to achieve long-term health and vitality.
One such traditional Ayurvedic remedy, Niruryadi Gulika (NG), stands out for its potent therapeutic properties. Composed of a blend of medicinal herbs selected for their synergistic effects, NG has been used for generations to address various health concerns, including digestive, respiratory, and neurological issues 12. NG is used for its anti-inflammatory and analgesic properties making it effective in managing conditions like joint pain & arthritis. Additionally, it promotes digestive health by alleviating symptoms of indigestion, bloating, and gas 13. Our research aims to investigate the properties of NG as a therapeutic agent in experimental diabetes. This investigation seeks to establish a foundation for integrating NG into modern therapeutic approaches for managing diabetes and its associated disorders.
Materials and Methods
Composition and Preparation of extracts
NG, a traditional Ayurvedic herbal formulation, consists of a synergistic blend of medicinal herbs and natural ingredients meticulously selected for their therapeutic properties. This unique composition includes herbs such as Nirgundi (Vitex negundo) containing Vitamin C, carotene, vitexin and many anti-inflammatory flavonoids 14, Triphala which contains many essential tannins, gallic acid, flavanoids, anthraquinones 15, Haridra (Curcuma longa L.) which is rich in curcuminoids such as Curcumin, desmethoxycurcumin and bisdemethoxy and diarylheptanoids, contribute significantly to the antioxidant and anti-inflammatory properties of NG 16 and Daruharidra (Berberis aristata) which contains alkaloids such as xyberberine, palmatine, karachine, aromoline, taxilamine and oxyacanthine 17 each known for their specific health benefits including anti-inflammatory, immunomodulatory and antioxidant effects 18-21. Additionally, ingredients like Danti (Baliospermum montanum Muell) containing Baliospermin and montanin, Ativisha (Aconitum heterophyllum) containing many alkaloids, and Chitraka (Plumbago zeylanica) constituting of plumbagin, coumarins and chitranone contribute to the formulation’s digestive and carminative properties, aiding in the alleviation of gastrointestinal discomfort. Guggulu (oleo-gum) resin obtained from the bark of Commiphora wightii and Shilajit (Asphaltum punjabianu) containing more than 20 minerals essential for the body further enhance the formulation with their cholesterol-lowering, anti-inflammatory, and rejuvenating properties, promoting overall strength and well-being. The plant parts used in the study were collected, shade-dried and extracted using a Soxhlet extractor. The resulting compound was then purified, distilled, and evaporated under vacuum and the remaining extract was stored at 2-8°C for further studies.
Experimental animals
Male Wistar rats, weighing approximately 150-200g on average, were used as experimental animals. They were housed in an acclimatized environment, with temperatures maintained between 22°C to 25°C, while adhering to a natural 12-hour photoperiod. The rats had access to water and were fed commercial rat feed (Ratan Brothers, India) ad libitum. All study procedures were conducted in accordance with the guidelines of the Institutional Animal Ethics Committee [Approval no IAEC/190/2022].
Induction of diabetes mellitus
To induce diabetes mellitus, the animals were initially divided into two groups. The first group (n = 6) was provided with a standard pelleted diet and served as the control, while the remaining rats (n = 40) were subjected to diabetes induction. Type 2 diabetes mellitus (T2DM) was induced in fasted rats by intraperitoneal (i.p.) injection of streptozotocin (STZ) at a dose of 40 mg/kg BW, dissolved in freshly prepared citrate buffer (pH 4.5)22. To prevent hypoglycemia from STZ administration, rats were given 5% glucose in their drinking water. After a three-day period, blood glucose levels of all experimental animals were measured using an Accu-Chek Instant Blood Glucose meter. Animals with blood glucose levels of ≥200 mg/dL were classified as diabetic and included in the study.
Experimental design
The animals were divided into six groups, each consisting of six rats, as follows: Group 1 – Untreated rats that received equivalent volume of the vehicle orally via gavage for 6 consecutive weeks; Group 2 – Diabetic control rats received the same volume of the vehicle orally via gavage for 6 weeks; Group 3 – Diabetic rats treated with NG at 100 mg/kg /BW/day via oral gavage for 6 weeks; Group 4 – Diabetic rats treated with NG at 200 mg/kg/BW/day via oral gavage for 6 weeks; Group 5- Diabetic rats treated with NG at 300 mg/kg/BW/day via oral gavage for 6 weeks; Group 6 – Reference drug, metformin was administered at a dose of 500-mg/kg /BW/day via oral administration for 6 weeks. NG and the reference drug, metformin, were administered to the animals via oral gavage during the study. No observable discomfort or agitation was noted following drug administration and there were no significant adverse effects on the wellness or behavioural parameters of the experimental animals.
Blood glucose levels were monitored throughout the duration of 6 weeks. At the end of the study, the rats were starved for 6 hrs, and euthanized following CPCSEA guidelines using the CO2 asphyxiation method. Serum was collected from whole blood by spinning at 2000 revolutions per minute for 5 minutes and was immediately stored in a deep freezer for subsequent analysis.
Measurement of glycated haemoglobin (HbA1C)
Haemoglobin A1c was estimated using a microcolumn (BioRad, CA, column size: 4.0 mm I.D × 30 mm, Model no: D-10), and reported as a percentage of total haemoglobin 23. Additionally, HbA1C was measured using affinity chromatography with aminophenyl boronic acid gels (Millipore, USA) to minimize potential interference from malondialdehyde-modified hemoglobin (MDA-Hb) in the estimation.
Assessment of Antioxidant Enzymes Catalase (CAT) and Glutathione Peroxidase (GPx)
For CAT, a reaction mixture was prepared by combining phosphate buffer and H₂O₂ solution to achieve a final H₂O₂ concentration of 0.1-0.3%. The reaction mixture was pre-warmed to 37°C. Sample dilutions were prepared using phosphate buffer, and the reaction was initiated by adding an appropriate volume of the sample to the pre-warmed reaction mixture. The mixture was immediately transferred to a cuvette or microcentrifuge tube and placed in a spectrophotometer. The decrease in absorbance was measured at 240 nm to estimate CAT activity 24.
For GPx, enzyme activity was assessed by measuring the consumption of NADPH during the reduction of oxidized glutathione (GSSG) catalyzed by glutathione reductase. One unit of GPx activity was defined as the amount of enzyme required to catalyze the formation of 1 micromole of reduced glutathione (GSH) per minute, corresponding to the oxidation of 0.5 micromole of NADPH per minute 25.
Estimation of TBARS
The standard solution of MDA was prepared by by mixing 100 µL of thiobarbituric acid reactive substances standard with 200 μL of TBARS Acid Reagent, gently agitating the mixture for 30 minutes to create a stock solution of 167 μM. A 16.7 μM solution act as the high standard, while deionized water was used as the blank. For the assay, 150 µL of samples and standards were pipetted into the microplate wells, followed by the addition of 75 µL of TBA reagent. The microplate was sealed with a cover strip and left undisturbed for 3 hrs at 45-50 °C and absorbance was estimated at 532 nm. The initial reading was subtracted from the final reading to calculate the unknown sample values 25.
Protocol for Lipid profile.
The enzymatic method utilizing cholesterol esterase and cholesterol oxidase was adopted to estimate the serum levels of total cholesterol (TC) 26,27. The HDL-c content in the supernatant was quantified in the supernatant after VLDL-c and LDL-c were precipitated using phosphotungstic acid and MgCl2 28. LDL-c and VLDL-c were calculated using Fried Ewald’s formula. Triglyceride levels were enzymatically estimated following hydrolysis by lipoprotein lipase, which generates glycerol-3-phosphate and dihydroxyacetone phosphate (DHAP) along with H2O2, which was then converted to amino phenazone in the presence of peroxidase for measurement 29.
Statistical analysis
All graphs and tables are presented as the mean ± SD. Statistical analyses were conducted using GraphPad Prism (Version 5.01, GraphPad Software). Group comparisons were evaluated using Student’s t-test, while multiple group analyses were performed with one-way ANOVA. Correlations between variables were assessed using Pearson’s correlation coefficient. A P-value of < 0.05 was considered statistically significant.
Results
Figure 1 shows haemoglobin, plasma insulin, glycated haemoglobin, and glucose levels in control and diabetic rats. The diabetic group exhibited significantly elevated glucose levels (### p < 0.001) compared to controls. Treatment with NG at all doses significantly reduced blood glucose levels in a dose-dependent manner (p < 0.001), demonstrating comparable efficacy observed in the metformin-treated group (p < 0.001).
Figure 1(b) shows total haemoglobin levels across the experimental groups. The diabetic control group showed a significant reduction in haemoglobin levels compared to the control group p <0.001). However, rats treated with NG demonstrated a dose-dependent increase in haemoglobin levels (p <0.001). Similarly, metformin-treated diabetic rats showed a significant increase in haemoglobin levels.
The diabetic control group exhibited significantly reduced plasma insulin levels compared to the control group p <0.001) [Figure 1(c)]. In contrast, the diabetic rats treated with NG established a marked increase in insulin levels, demonstrating a similar trend as observed in the metformin-treated diabetic rats p <0.001).
Figure 1(d) illustrates the HbA1c levels across the study groups. Diabetic control rats showed an approximately 0.7-fold increase in HbA1c levels compared to the control group (p <0.001). In contrast, all NG treated diabetic rats exhibited a significant dose-dependent reduction in HbA1c levels (p <0.001). Similarly, diabetic rats treated with metformin exhibited a marked decrease in HbA1c levels compared to the diabetic group.
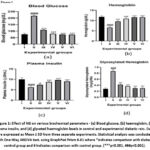 |
Figure 1: Effect of NG on various biochemical parameters – (a) Blood glucose, (b) haemoglobin, (c) Plasma insulin, and (d) glycated haemoglobin levels in control and experimental diabetic rats.Click here to view Figure |
Figure 2 depicts the effect of NG on the levels of inflammatory cytokine, TNF-α and IL-6, across the experimental groups. The cytokine levels were significantly elevated in the diabetic group compared to the control group. However, NG-treated diabetic rats exhibited a significant reduction in these cytokines, in a dose-dependent manner. Notably, the TNF-α levels in the 300 mg/kg NG-treated group were comparable to those observed in the metformin-treated rats, highlighting the potential anti-inflammatory properties of NG.
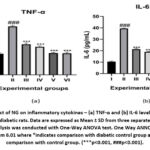 |
Figure 2: Effect of NG on inflammatory cytokines – (a) TNF-α and (b) IL-6 levels control and experimental diabetic rats.Click here to view Figure |
Figure 3 shows the effect of NG on lipid profile markers, indicating that triglycerides were significantly elevated in diabetic animals (### p <0.001), while NG treatment resulted in a dose-dependent decrease. Total cholesterol levels also elevated in the diabetic group, were markedly reduced in the NG-treated groups (p <0.001), with reductions comparable to those observed in the metformin treatment. HDL-c levels were significantly reduced in the diabetic control group (p <0.001) but increased with NG treatment and metformin. LDL-c levels were higher in the diabetic control group and reduced in all NG-treated groups, showing similar reductions to the metformin-treated rats (p <0.001).
 |
Figure 3: Effect of NG on lipid profile markers (a) Triglycerides, (b) Total Cholesterol, (c) HDL-c and (d) LDL-c in the control and experimental diabetic rats.Click here to view Figure |
Figure 4 depicts the levels of TBARS, a marker of lipid peroxidation, which was significantly elevated in the diabetic group (p <0.001). Treatment with NG resulted in a dose-dependent reduction in TBARS levels, with similar effects observed in the metformin-treated rats.
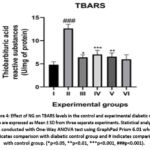 |
Figure 4: Effect of NG on TBARS levels in the control and experimental diabetic rats.Click here to view Figure |
Figure 5 highlights the significant decrease in antioxidant enzyme activity, specifically catalase and glutathione peroxidase (GPx), in the diabetic control group compared to the control group (### p < 0.001). NG treatment led to a dose-dependent increase in these antioxidant enzyme levels, with the 300 mg/kg NG-treated group showing the maximum improvement in catalase activity (p < 0.01). Similarly, both catalase and GPx levels were significantly elevated in the metformin-treated rats, reflecting enhanced antioxidant defense.
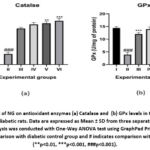 |
Figure 5: Effect of NG on antioxidant enzymes (a) Catalase and (b) GPx levels in the control and experimental diabetic rats.Click here to view Figure |
Discussion
Diabetes mellitus is a global health issue with a substantial impact, affecting approximately 500 million adults worldwide, with projections estimating an increase to over 750 million by 2045 30. This alarming rise underscores the urgent need for effective management and treatment strategies. Conventional diabetes treatments often have adverse effects, prompting researchers to explore alternative therapies with improved safety profiles. Herbal medicines have garnered increasing attention due to their minimal side effects and enhanced therapeutic potential 31,32. This study investigates the anti-diabetic and antioxidative role of NG in streptozotocin (STZ)-induced diabetic rats.
STZ induces diabetes by selectively destroying pancreatic beta cells, leading to decreased plasma insulin levels and elevated blood glucose 22,33. It damages DNA through alkylation in β cells by entering via the GLUT2 transporter, generating ROS, and releasing nitric oxide, which causes oxidative stress 34. This oxidative stress overwhelms the cell’s antioxidant defences, resulting in cellular damage and triggering apoptosis and necrosis 35. Additionally, STZ induces inflammation, releasing pro-inflammatory cytokines, which are major contributors for beta cell death 36,37.
The combination of DNA damage, redox imbalance, and mitochondrial dysfunction results in significant β-cell loss, leading to a dramatic reduction in insulin secretion. This, in turn, results in hyperglycemia, mimicking the pathophysiological characteristics of diabetes, and thus provids a valuable model for studying the disease and potential treatments 22. STZ also adversely affects lipid metabolism, increasing TG, LDL, and total lipid levels while decreasing HDL levels 38,39. Moreover, STZ disrupts the redox balance, contributing to oxidative stress and further beta cell damage 40,41. Our findings align with these previous studies, as the diabetic control group showed elevated blood glucose and haemoglobin levels, decreased plasma insulin and total haemoglobin levels, increased TNF-α and IL-6 levels, abnormal lipid profiles, and heightened lipid peroxidation. Treatment with NG significantly ameliorated these adverse effects in a dose-dependent manner.
NG led to a marked reduction in glucose and haemoglobin levels, demonstrating its potent anti-diabetic properties. The most substantial reduction was observed in the rats administered 300 mg/kg NG. This group also displayed a significant increase in plasma insulin levels, indicating improved beta-cell function or enhanced insulin sensitivity. The dose-dependent response suggests that higher doses of NG might have a more pronounced therapeutic effect, although future findings are essential to confirm this.
Inflammatory cytokines play critical roles in the development of diabetes and its complications by contributing to beta cell destruction 42,43. Our study revealed that NG treatment significantly downregulated these cytokines. Notably, the 300 mg/kg NG-treated rats exhibited cytokine levels comparable to those in metformin-treated rats. This anti-inflammatory effect of NG may occur through the suppression of the NF-KB pathway, a known mechanism underlying metformin’s anti-inflammatory action 44,45.
Diabetes is often associated with dyslipidaemia, and an elevated lipid profile 46,47. In our study, NG treatment normalized these lipid abnormalities, with the 300 mg/kg dose showing the most significant improvements. These findings suggest that NG may help mitigate the cardiovascular risks associated with diabetes by improving lipid metabolism.
Lipid peroxidation, indicated by elevated TBARS levels, is a common consequence of diabetes-induced oxidative stress 48,49. NG treatment significantly reduced TBARS levels in diabetic rats, indicating its potential to inhibit lipid peroxidation. This effect is crucial as it helps in protecting cellular membranes from oxidative damage, thereby preserving cell integrity and function.
Antioxidant genes such as catalase and GPx play vital roles in combating oxidative stress. Our study found that NG treatment significantly elevated these enzymes in diabetic rats. The 300 mg/kg NG-treated group exhibited particularly high levels of catalase, suggesting a potent antioxidative response. This enhancement of antioxidant defences aligns with previous studies highlighting the role of dietary antioxidants in improving insulin responses and overall diabetic status 50,51.
The exact mechanisms through which NG exerts its anti-diabetic and antioxidative effects remain to be fully elucidated. However, it is plausible that NG enhances insulin secretion or sensitivity, modulates inflammatory pathways, improves lipid metabolism, inhibits lipid peroxidation, and boosts antioxidant defense mechanism. Further research, including molecular studies, is needed to uncover the precise pathways involved.
The findings of this study have significant clinical implications. NG, a natural compound, shows promise as a potential treatment for diabetes management, offering a safer alternative to conventional drugs with fewer side effects. Future studies should focus on clinical trials to validate these findings in humans. Additionally, exploring the synergistic effects of NG with other anti-diabetic agents could provide valuable insights into more effective combination therapies.
Conclusion
In conclusion, our research supports the role of NG as a potent anti-diabetic formulation, with beneficial effects on cellular antioxidant response and lipid peroxidation. NG treatment moderated plasma insulin levels, reduced high blood glucose, restored lipid profiles, lowered TBARS levels, and enhanced antioxidant enzyme activity in STZ-induced diabetic rats. These findings highlight the potential of NG in diabetes management and emphasize the need for further investigation into its mechanisms of action and clinical applications.
Acknowledgment
The authors gratefully acknowledge the Interdisciplinary Institute of Indian System of Medicine, SRM Institute of Science and Technology, Kattankulathur, for its support.
Conflict of Interest
The author(s) declare no conflict of interest
Funding Sources
The author(s) received no financial support for the research authorship, and/or publication of this article.
Data availability Statement
This statement does not apply to this article
Ethical Approval
All study procedures were accomplished in agreement with Institutional Animal Ethics Committee guidelines [Approval no IAEC/190/2022]. This study did not involve human participants, so informed consent was not required.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials
Author Contributions
Conceptualization, R.C.S, C.D., K.M.R.;
Methodology and analysis, R.C.S, M.B.;
Investigation, R.C.S, M.B.;
Writing—original draft preparation, R.C.S, M.B..;
Writing-review and editing, K.M.R., C.D.;
Funding – R.C.S, C.D.;
Supervision, C.D.
References:
- Reaven GM. Banting lecture. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595-607.
CrossRef - Krause M, De Vito G. Type 1 and Type 2 Diabetes Mellitus: Commonalities, Differences and the Importance of Exercise and Nutrition. Nutrients. 2023;15(19).
CrossRef - Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813-20.
CrossRef - Rolo AP, Palmeira CM. Diabetes and mitochondrial function: Role of hyperglycemia and oxidative stress. Toxicology and Applied Pharmacology. 2006;212(2):167-178.
CrossRef - Petersen MC, Shulman GI. Mechanisms of Insulin Action and Insulin Resistance. Physiological Reviews. 2018;98(4):2133-2223.
CrossRef - Jiang S, Young JL, Wang K, Qian Y, Cai L. Diabetic‑induced alterations in hepatic glucose and lipid metabolism: The role of type 1 and type 2 diabetes mellitus (Review). Mol Med Rep. 2020;22(2):603-611.
CrossRef - Haeusler RA, McGraw TE, Accili D. Biochemical and cellular properties of insulin receptor signalling. Nature Reviews Molecular Cell Biology. 2017;19(1):31-44.
CrossRef - Gordon A, Buch Z, Baute V, Coeytaux R. Use of Ayurveda in the Treatment of Type 2 Diabetes Mellitus. Global Advances in Health and Medicine. 2019;8.
CrossRef - Modak M, Dixit P, Londhe J, Ghaskadbi S, Devasagayam TPA. Indian Herbs and Herbal Drugs Used for the Treatment of Diabetes. Journal of Clinical Biochemistry and Nutrition. 2007;40(3):163-173.
CrossRef - Bindu J, Narendhirakannan RT. Role of medicinal plants in the management of diabetes mellitus: a review. 3 Biotech. 2018;9(1).
CrossRef - Panda C, Sharma P, Dixit US, Pandey LM. Potential and Prospective of Traditional Indian Medicinal Plants for the Treatment of Diabetes. Journal of Biologically Active Products from Nature. 2023;13(4):316-360.
CrossRef - Parameswaran K, Mahapatra A, Shrikrishna R, Ojha N, Dharmarajan P, Dileep A. Prameha (diabetes): A scoping review of updates from Keraliya Ayurveda literature. Journal of Indian System of Medicine. 2023;11(1).
CrossRef - Vaibhav B, S K. Critical Review on different herbs of Niruryādi Guḷika for their Anti-Diabetic properties.2017;3:1500-1508.
- Kannojia P, Mishra P, Singh Y. Morphology, Phytochemistry and Pharmacological Activity of Vitex negundo: An Overview. Journal of Drug Delivery and Therapeutics. 2020;10(3-s):280-285.
CrossRef - Belapurkar P, Goyal P, Tiwari-Barua P. Immunomodulatory effects of triphala and its individual constituents: a review. Indian J Pharm Sci. 2014;76(6):467-75.
- Rout KK, Parida S, Mishra SK. Standardization of the ayurvedic formulation Haridra Khanda using high-performance thin-layer chromatography-densitometry. J AOAC Int.2008;91(5):1162-8.
CrossRef - Potdar D, Hirwani RR, Dhulap S. Phyto-chemical and pharmacological applications of Berberis aristata. Fitoterapia.2012;83(5):817-30.
CrossRef - Peterson CT, Denniston K, Chopra D. Therapeutic Uses of Triphala in Ayurvedic Medicine. The Journal of Alternative and Complementary Medicine. 2017;23(8):607-614.
CrossRef - Shailja C, Hemlata K, Madhusudan S, Gitika C. Daruharidra (Berberis aristata): Review based upon its Ayurvedic Properties. International Journal for Research in Applied Sciences and Biotechnology. 2021;8(2):98-106.
CrossRef - D′Mello P, Kulkarni RR, Virkar AD. Antioxidant and antiinflammatory activity of Vitex negundo. Indian Journal of Pharmaceutical Sciences. 2008;70(6).
CrossRef - Fuloria S, Mehta J, Chandel A. A Comprehensive Review on the Therapeutic Potential of Curcuma longa Linn. in Relation to its Major Active Constituent Curcumin. Frontiers in Pharmacology. 2022;13.
CrossRef - Ghasemi A, Jeddi S. Streptozotocin as a tool for induction of rat models of diabetes: a practical guide. EXCLI J. 2023;22:274-294.
- Kulkarni JD, Shivashanker S. Incidental Detection of Hemoglobin Variants During Evaluation of HbA1c. Indian J Clin Biochem. 2022;37(2):242-246.
CrossRef - Hadwan MH. Simple spectrophotometric assay for measuring catalase activity in biological tissues. BMC Biochem.2018;19(1):7.
CrossRef - Baud O, Greene AE, Li J, Wang H, Volpe JJ, Rosenberg PA. Glutathione peroxidase-catalase cooperativity is required for resistance to hydrogen peroxide by mature rat oligodendrocytes. J Neurosci.2004;24(7):1531-40.
CrossRef - Kamelska AM, Jarmołowska B, Bryl K. A simplified enzymatic method for total cholesterol determination in milk. International Dairy Journal. 2015;50:50-57.
CrossRef - Malik V, Pundir CS. Determination of total cholesterol in serum by cholesterol esterase and cholesterol oxidase immobilized and co-immobilized on to arylamine glass. Biotechnol Appl Biochem. 2002;35(3):191-7.
CrossRef - Schmitz G, Assmann G. Isolation of human serum HDL1 by zonal ultracentrifugation. Journal of Lipid Research. 1982;23(6):903-910.
CrossRef - Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem.1982;28(10):2077-80.
CrossRef - Magliano DJ, Boyko EJ. IDF DIABETES ATLAS. 10th ed. 2021. IDF Diabetes Atlas.
- Ruiz-Noa Y, Ibarra-Reynoso LDR, Ruiz-Padilla AJ. Use of herbal medicine for diabetes mellitus in adults from the central–western region of Mexico. Primary Care Diabetes. 2021;15(6):1095-1099.
CrossRef - Akhtar MS, Rafiullah M, Hossain MA, Ali M. Antidiabetic activity of Cichorium intybus L water extract against streptozotocin-induced diabetic rats. Journal of Umm Al-Qura University for Applied Sciences. 2023;9(4):565-571.
CrossRef - Mythili MD, Vyas R, Akila G, Gunasekaran S. Effect of streptozotocin on the ultrastructure of rat pancreatic islets. Microsc Res Tech. 2004;63(5):274-81.
CrossRef - Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50(6):537-46.
CrossRef - Lenzen S. Oxidative stress: the vulnerable beta-cell. Biochem Soc Trans. 2008;36(Pt 3):343-7.
CrossRef - Arokoyo DS, Oyeyipo IP, Du Plessis SS, Chegou NN, Aboua YG. Modulation of Inflammatory Cytokines and Islet Morphology as Therapeutic Mechanisms of Basella alba in Streptozotocin-Induced Diabetic Rats. Toxicol Res.2018;34(4):325-332.
CrossRef - Roy S, Metya SK, Sannigrahi S, Rahaman N, Ahmed F. Treatment with ferulic acid to rats with streptozotocin-induced diabetes: effects on oxidative stress, pro-inflammatory cytokines, and apoptosis in the pancreatic beta cell. Endocrine.2013;44(2):369-79.
CrossRef - Andallu B, Vinay Kumar AV, Varadacharyulu N. Lipid abnormalities in streptozotocin-diabetes: Amelioration by Morus indica L. cv Suguna leaves. Int J Diabetes Dev Ctries.2009;29(3):123-8.
CrossRef - Dennis A, E OU, E EE, E EA, I EM. Lipid profile status of streptozotocin induced diabetic rats treated with ethanolic leaf extract of Solenostemon monostachyus. Journal of Medicinal Plants Research. 2015;9(8):289-293.
CrossRef - Gomathi D, Kalaiselvi M, Ravikumar G, Devaki K, Uma C. Evaluation of Antioxidants in the Kidney of Streptozotocin Induced Diabetic Rats. Indian Journal of Clinical Biochemistry. 2013;29(2):221-226.
CrossRef - Sharma P, Singh R, Bhardwaj P. Antioxidant and toxicological evaluation of Cassia sopherain streptozotocin-induced diabetic Wistar rats. Pharmacognosy Research. 2013;5(4).
CrossRef - Mabrouk A A-Z, Soha S M, Ahmed H I. Evaluation of Some Inflammatory Cytokines Levels as A Marker for Diabetes. International Journal of Immunology and Immunotherapy. 2022;9(1).
CrossRef - Yagihashi S. Contribution of animal models to diabetes research: Its history, significance, and translation to humans. Journal of Diabetes Investigation. 2023;14(9):1015-1037.
CrossRef - Cao X-J, Wu R, Qian H-Y. Metformin attenuates diabetic neuropathic pain via AMPK/NF-κB signaling pathway in dorsal root ganglion of diabetic rats. Brain Research. 2021;1772.
CrossRef - Pon Velayutham AB, Li Y, Gappy S. Metformin suppresses pro-inflammatory cytokines in vitreous of diabetes patients and human retinal vascular endothelium. Plos One. 2022;17(7).
CrossRef - Jayakumari C, Jabbar PK, Soumya S. Lipid Profile in Indian Patients With Type 2 Diabetes: The Scope for Atherosclerotic Cardiovascular Disease Risk Reduction. Diabetes Spectrum. 2020;33(4):299-306.
CrossRef - Sabahelkhier MK, Awadllah MA, Mohammed Idrees AS, Abel Rahheem Mohammed AA-G, Idris MAR. A study of lipid profile Levels of Type II Diabetes Mellitus. Nova Journal of Medical and Biological Sciences. 2016;5(2).
CrossRef - Turk HM, Sevinc A, Camci C. Plasma lipid peroxidation products and antioxidant enzyme activities in patients with type 2 diabetes mellitus. Acta Diabetologica. 2014;39(3):117-122.
CrossRef - Shabalala SC, Johnson R, Basson AK. Detrimental Effects of Lipid Peroxidation in Type 2 Diabetes: Exploring the Neutralizing Influence of Antioxidants. Antioxidants. 2022;11(10).
CrossRef - Akbar S, Bellary S, Griffiths HR. Dietary antioxidant interventions in type 2 diabetes patients: a meta-analysis. The British Journal of Diabetes & Vascular Disease. 2011;11(2):62-68.
CrossRef - She C, Shang F, Cui M, Yang X, Liu N. Association between dietary antioxidants and risk for diabetic retinopathy in a Chinese population. Eye. 2020;35(7):1977-1984.
CrossRef







