Suman Ghosh1 , Ishita Debnath1
, Ishita Debnath1 , Sobhanjan Bhunia1
, Sobhanjan Bhunia1 , Supriya Hazra1
, Supriya Hazra1 , Sumit Nandi2*
, Sumit Nandi2* , Sudip Kumar Mandal3
, Sudip Kumar Mandal3 , Sneha Mallya4
, Sneha Mallya4 , Souvik Chakraborty4
, Souvik Chakraborty4 , Aditya Raj Shaw4
, Aditya Raj Shaw4 , Soumyadip Patra4
, Soumyadip Patra4 and Rajia Khatun4
and Rajia Khatun4
1Department of Pharmaceutical Chemistry, Guru Nanak Institute of Pharmaceutical Science and Technology, Kolkata, West Bengal, India
2Department of Pharmacology, Gupta College of Technological Sciences, Asansol, West Bengal, India
3Department of Pharmaceutical Chemistry, Dr. B. C. Roy College of Pharmacy and Allied Health Sciences, Durgapur, West Bengal, India
4Department of Pharmaceutical Technology, Bharat Technology, Howrah, West Bengal, India
Corresponding Author E-Mail: sumitrx27@gmail.com
Abstract
Histamine-mediated allergic reactions are central to the pathogenesis of various allergic disorders, involving complex molecular mechanisms. Cetirizine, a second-generation antihistamine, functions as a potent H1 receptor antagonist and is widely utilized for the management of these conditions. This review comprehensively evaluated the molecular mechanisms underlying histamine release, its interaction with H1 receptors, and the subsequent allergic responses. It also analyzed the pharmacokinetics and pharmacodynamics of cetirizine, highlighting its high selectivity for H1 receptors and its minimal sedative effects. The clinical efficacy of cetirizine was demonstrated across several allergic conditions, including food allergies, atopic dermatitis, allergic conjunctivitis, and drug-induced hypersensitivities. In these contexts, cetirizine reduced histamine-mediated symptoms, such as pruritus and inflammation, and improved patient outcomes. Additionally, combination therapies involving cetirizine with other antihistamines, corticosteroids, or biologics were discussed, particularly in refractory or severe allergic cases. Despite its overall safety, this review also highlighted the adverse effects associated with long-term cetirizine use, particularly in special populations such as pediatric, elderly, and patients with renal impairment. Future perspectives emphasize the role of future research on cetirizine in personalized allergy treatment, emerging combination therapies with biologics, and its potential in prophylactic applications.
Keywords
Cetirizine; Clinical efficacy; Histamine; H1 receptor antagonist; Pharmacodynamics; Pharmacokinetics
| Copy the following to cite this article: Ghosh S, Debnath I, Bhunia S, Hazra S, Nandi S, Mandal S. K, Mallya S, Chakraborty S, Shaw A. R, Patra S, Khatun R. A Review on Mechanism of Histamine Mediated Allergic Reactions: Therapeutic Role, Safety, and Clinical Efficacy of Cetirizine in Modern Allergy and Other Diseases Management. Biomed Pharmacol J 2025;18(1). |
| Copy the following to cite this URL: Ghosh S, Debnath I, Bhunia S, Hazra S, Nandi S, Mandal S. K, Mallya S, Chakraborty S, Shaw A. R, Patra S, Khatun R. A Review on Mechanism of Histamine Mediated Allergic Reactions: Therapeutic Role, Safety, and Clinical Efficacy of Cetirizine in Modern Allergy and Other Diseases Management. Biomed Pharmacol J 2025;18(1). Available from: https://bit.ly/4jSv9T7 |
Introduction
Cetirizine is a second-generation antihistamine primarily used to manage allergic reactions. Allergy development is a multifactorial process that begins during sensitization when particular allergens cause the formation of IgE antibodies. These IgE antibodies cause degranulation, which releases a number of mediators, including histamine, by binding to the high-affinity receptors (FcεRI) found on mast cells, basophils, and antigen-presenting cells (APCs) 1,2. This process triggers immediate hypersensitivity reactions, which manifest as common allergic symptoms. Inducing allergic inflammation largely depends on effector Th2 cells, which release cytokines like IL-4, IL-5, and IL-13, which promote eosinophilia, mucus secretion, and recruitment of inflammatory cells, thereby exacerbating allergic responses 3–5. Eosinophils and mast cells are crucial for the initiation and progression of allergic reactions, and histamine is one of the main mediators of acute hypersensitivity. In contrast, eosinophils contribute to chronic inflammation through the release of cytotoxic proteins 6–8. Histamine release is a crucial phase in allergic responses, occurring when intracellular histamine stored in mast cell granules is released into the extracellular environment. This is initiated by antigen-antibody interactions, where FcεRI-bound IgE on mast cells stimulates tyrosine-protein kinase activation. This cascade leads to the phosphorylation of phospholipase Cγ and the subsequent intracellular calcium release, which facilitates granule fusion with the plasma membrane, releasing histamine. Histamine, synthesized from histidine by the enzyme L-histidine decarboxylase, acts as a potent signaling molecule by binding to H1, H2, H3, and H4 receptors, all belong to the GPCR family 9–11. These receptors mediate various physiological and pathological processes, with H1 receptor activation playing a key role in allergic symptoms. Upon binding to the H1 receptor, histamine triggers a cascade involving Gq/11 protein activation and phospholipase C signaling, producing DAG and IP3. This promotes smooth muscle contraction, increases vascular permeability, as well as contributes to the hallmark symptoms of allergic responses, such as itchiness, bronchoconstriction, and tissue swelling. Cetirizine’s action as an H1 receptor antagonist effectively blocks this pathway, reducing the effects of histamine and alleviating allergy symptoms 12,13. Cetirizine relieves symptoms associated with allergies, such as itching, swelling, and rhinorrhea, without causing the sedative effects commonly observed with first-generation antihistamines. Studies have demonstrated its effectiveness not only in traditional allergic conditions like hay fever and urticaria but also in newer allergies such as food allergies, atopic dermatitis, and allergic conjunctivitis 14,15. Furthermore, cetirizine has shown a favourable safety profile in long-term use, making it suitable for a broad spectrum of patients with chronic allergic conditions. This review aims to provide a thorough analysis of the mechanisms underlying histamine-mediated allergic reactions, the molecular and pharmacological actions of cetirizine, and its clinical efficacy and safety profile, particularly in emerging allergic conditions.
Causes of allergy formation
Inflammatory mediators and a variety of cell types interact to produce an immunological cascade of allergy diseases. Sensitization, early-phase reactions, and late-phase responses are the three separate stages of the allergic response. Producing IgE-antibodies specific to allergens, which adhere to mast cell, basophil, and APCs surfaces to initiate degranulation and mediator release, is the initial stage of the sensitization phase. After that, allergen-specific CD4+Th2 cells undergo clonal proliferation and differentiation, enabling them to produce IL-4 and IL-13, two important factors of IgE production 16. IgE attaching to effector cells makes a patient more sensitive to a specific allergen. In the initial stages of the reaction, histamine, tryptase, eosinophil chemotactic factor, and newly synthesized molecules (PGD2, LCT4, bradykinin) are secreted by basophiles and mast cells 17. This explains why patients who demonstrate a preliminary allergy response also undergo a late-phase inflammatory response 4 to 24 hours after being exposed to allergens. A recurrence of the early-stage symptoms occurs during the later stage of the allergy response, which is marked by tissue damage as well as inflammation. Allergy disorders refer to the complex immune system responses to environmental antigens that result in inflammatory reactions with a prevalence of IgE specific to allergens and a T-helper-2 type cell. In essence, allergy is an inflammatory illness 18. Recent years have seen a substantial advancement in our knowledge of the cells and mediators that affect allergic inflammation. This information serves as the cornerstone for the more logical formulation of treatment concepts and the mitigation of allergy symptoms. Many different cells are involved in allergic inflammation. Three distinct cell types, though, appear to be particularly significant. These are the T-lymphocyte Th2-type, the mast cell, and the eosinophil granulocyte. Naive T cells differentiate into a variety of subsets, such as Th1, Th2, Th9, Th17, and Th22 type memory and effector cells, in large part due to cytokines, other substances, and the cells in the microenvironment 19. In the presence of allergic diseases, effector Th2 cells produce proinflammatory cytokines such as IL-25, IL-31, and IL-33 in addition to more common Th2 cytokines including IL-4, IL-5, IL-9, and IL-13. These cytokines sometime may cause mucus production, eosinophilia, the generation of IgE specific to allergens, as well as migration of inflammatory cells to inflammatory tissues. Primary effector cells are eosinophilia and mast cells in this aspect. Histamine release is one of the primary consequences of mast cell activation, which causes an immediate allergic reaction (Figure 1). When eosinophils are activated, several strong cytotoxic proteins are released extracellularly. The development of subacute and chronic allergy symptoms is significantly influenced by these proteins. In addition to macrophages, endothelial cells, neutrophil granulocytes, and epithelial cells are also crucial in allergic disorders 20.
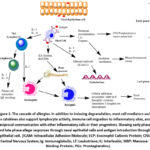 |
Figure 1: The cascade of allergies. In addition to inducing degranulation, mast cell mediators such as cytokines also support lymphocyte activity, immune cell migration to inflammatory sites, and reciprocal communication with other inflammatory cells or their progenitors. |
Mechanism involved in histamine release
Histamine is produced and released by various human cells, including lymphocytes, enterochromaffin cells, basophils, mast cells, platelets, and histaminergic neurons. Within intracellular granules of mast cells, histamine is retained by heparin and an acidic protein. Sodium ions (Na+) in the extracellular fluid exchange with histamine during granule extrusion via exocytosis, thus histamine releases 21.
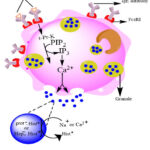 |
Figure 2: Mechanism of Histamine release |
The mechanism by which mast cells release histamine in response to an antigen-antibody reaction. Sensitized atopic individuals produce specific reaginic antibodies (IgE), which attach to the Fc epsilon receptor (FcɛRI) on mast cell surfaces. When the antigen bridge IgE molecules are challenged, tyrosine-protein-kinase (t-Pr-K) is transmembrane activated and phosphorylates phospholypase C𝛄. Inositol triphosphate (IP3) is produced upon hydrolysis of phosphotidyl inositol biphosphate (PIP2), which causes the calcium ion to be released between cells. Granule content is released exocytotically when the calcium ion causes the granule membrane to fuse with the mast cell’s plasma membrane. The granule contained a complex of negatively charged protein and heparin molecules with positively charged histamine. Histamine is released to act on target cells through cationic exchange with extracellular sodium. It is kept in granules or vesicles that are released upon activation. Dale and Laidlaw discovered histamine [2-(4-emidazolyl) ethylamine] in 1910, and in 1932 they ascertained that it was an intermediate of anaphylactic reactions 22. Histidine is converted to histamine, one of the biogenic amines, by the enzyme L-histidine decarboxylase (HDC), which requires pyridoxal phosphate (vitamin B6). A powerful modulator of many physiological responses is histamine. There are many types of histamine receptors like H1, H2, H3, H4, etc. Histamine is a biogenic amine. H1-receptor is a part of the GPCR superfamily. This superfamily is made up of almost 500 distinct membrane proteins that are connected by a structural pattern consisting of seven transmembrane helical regions. Histamine has various physiological and pathophysiological effects on histamine receptors 23. Another recent study found that a protein called caspase-8 may play a role in the release of histamine from mast cells. Caspase-8 is an enzyme that is involved in cell death. The study found that mice lacking caspase-8 had increased levels of histamine in their blood.
Effect of histamine on H1 receptor
As H1 receptor binding induces smooth muscle contraction and vascular permeability H1 antagonist is the best choice for alleviating the effect of allergies. The human chromosome 3 encodes the H1 receptor, which is in charge of several symptoms and indicators of allergic diseases, including itchiness, rhinorrhea, asthma, and stiffness of the intestinal smooth muscle 24. H1 activation is associated with Gq/11 GTP-hydrolysing protein that activates phospholipase C, then histamine binds with the H1 receptor. In turn, phospholipase C hydrolyzes phosphatidylinositol-4,5-bisphosphate to produce DAG and IP3, two more second messengers. DAG potentiates, and inositol phospholipid signaling pathways are stimulated. The activity of PKC releases stored Ca2+ ions into the cytoplasm through IP3 binding on the endoplasmic reticulum. Moreover, H1 stimulation can also activate two additional intracellular signaling pathways phospholipase D and A. It has recently been demonstrated that nuclear transcription factor κB (NFκB) can be activated by heme-regulated inhibitor (HRI) activation. Both contribute to the emergence of allergic illnesses. In the process of figuring out how histamine affects stomach acid secretion, the H2 receptor was discovered. After being cloned in dogs, it was subsequently discovered in various species. The H2 receptor is encoded by an intronless gene and has a protein with 358 to 359 amino acids. It is connected to both PKA and Gs 25.
The somata, dendrites, and axons (varicosities) of histaminergic and other cells have H3 receptors, which function as a negative feedback loop to restrict the production and release of histamine as well as other transmitters such as acetylcholine, noradrenaline, and glutamate. Gi/o and high voltage-triggered Ca2+ channels, a common method for controlling transmitter release, are related to H3 receptors. The H3 receptor triggers the MAPK pathway by being negatively linked to cAMP.
Molecular mechanism of histamine
Histamine has a major impact on the CNS is now apparent because it was discovered that traditional antihistamines have sedative effects. In almost all animals’ histamine is released in brain cell 26. However, the histamine content in cells varied between higher to lower vertebrates. In lower vertebrates, histamine is present in higher amounts because of their lower-developed cortex and cerebellum. Histamine-producing neurons can be found in the tuberomamillary nucleus (TMN), which is a component of the posterior hypothalamus in both humans and animals. Although the primary terminals of histamine secretors vary among species, they are all found in crucial regions of the CNS 27. All mammals have moderate to intense histaminergic innervation in the cerebral cortex, amygdala, substantia nigra, and striatum. The tuberomammillary nucleus also sends histaminergic fibers to the retina and spinal cord, and the density of these projections in the thalamus and hippocampal regions varies. The afferent projection of the tuberomammillary nucleus comes from many areas among them the prominent source is the infralimbic cortex, lateral septum, preoptic nucleus. The primary sources of brainstem stimulation are the noradrenergic groups A1 to A3, the adrenergic cell groups C1 to C3, and the serotonergic groups B5 to B9. Only a few numbers of fibers, however, pass through the dopaminergic groups of the ventral tegmental area, substantia nigra, as well as locus coeruleus to reach the TM nucleus 28.
Neurons use histamine synthesis and inactivation as the primary mechanisms for histamine transport and metabolism. The process is that when histidine is released into the body it enters neuron cells by L-amino-acid transporter. Histidine decarboxylase in neurons converts histidine into histamine. Formed histamine is brought into vesicles by VMAT-2. After the formation of histamine, inactivation of histamine occurs postsynaptically. After the release of histamine from VMAT-2 methylation occurs in postsynaps by Histamine methyltransferase and form Tele-methylhistamine. Tele-methylhistamine is a substance that has no effects like histamine. This methylation process is the main inactivation process of histamine 29. Neuronal histamine has a relatively high turnover rate and a half-life that varies rapidly based on neuronal activity, usually lasting around 30 minutes.
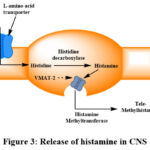 |
Figure 3: Release of histamine in CNS |
Pharmacokinetic profile of Cetirizine
The oral dose has rapid absorption in the gut. The maximum plasma concentration achieved by cetirizine is 1-2 hours. The bioavailability of this drug is the same for any dosage formulation (tablet, syrup). There is no difference in the amount of absorption between the fed and fasted states, despite the possibility that the presence of food will accelerate absorption rate. The majority of the medication roughly 90% is bonded to albumin and other plasma proteins. Oral cetirizine is quickly absorbed into breast milk and tear fluid in allergic conjunctivitis patients; nevertheless, the medication is not easily absorbed through the blood-brain barrier. Elimination of cetirizine occurs via urine (70% of administered drug) rest small quantity is eliminated by faeces.
Pharmacodynamic profile of Cetirizine
Cetirizine, the carboxylate metabolite of hydroxyzine, is a zwitterion that may help to explain some of its characteristics, including its limited biotransformation potential, low to moderate lipophilicity, strong hydrogen-binding capacity, and restricted blood-brain barrier penetration. With very little affinity for several other receptor types, such as adrenergic and serotoninergic receptors, it is an extremely selective antagonist of the histamine H1 receptor 30. In non-atopic and atopic adults as well as pediatric patients, oral cetirizine 10 mg administered once or more times suppressed histamine-induced wheal and flare reactions. Additionally, this drug shows a higher level of efficacy in comparison to therapeutic dosages of other antihistamine medications, including loratadine or ebastine. Cetirizine’s effects lasted for at least 24 hours, peaking between 4-8 hours in. Cetirizine 5-20 mg offered dose-dependent defense against histamine-induced respiratory. Despite reports of somnolence in clinical trials, cetirizine 10 mg once daily did not typically impair CNS function. Cetirizine did not affect an adult’s cognitive performance, a pediatric patient’s behavior, or their psychomotor milestones. Since cetirizine binds to histamine H1-receptors, many of the effects of histamine are effectively reversed.
Mechanism of action of Cetirizine
Although second generation H1 antihistamines are derived from first-generation H1 antihistamines, they have more benefits than the first generation of drugs because they have fewer sedative or anticholinergic effects. They do, however, also have negative effects, and some of them combine with other medications and substances. Cetirizine, a carboxylic acid with a racemic mix of R and S enantiomers, is derived from hydroxyzine. It is not metabolized in the liver and does not interact with any inducers or inhibitors of the CYP (cytochrome p450 system) 31. When cetirizine is given up to six times the recommended dosage, no changes have been seen in electrocardiography. Since cetirizine seems to be a P glycoprotein (Pgp) substrate, pharmacological interactions at this level are conceivable but not fully understood. When a person ages and develops renal or hepatic disorders, they must change their dosage. The sedative effects of traditional antihistamines, which function as H1 antagonists, are widely recognized. Cellular excitation is accomplished through Gq/11 and PLC activation, resulting in the formation of diacylglycerol (DAG) and Inositol 1,4,5-triphosphate [Ins(1,4,5)P3], the two-second messengers. Inositol 1,4,5-triphosphate release stimulates dye-coupling via gap junctions and causes the supraoptic nucleus to express C-Fos 32. Within this nucleus, neurons expressing vasopressin have been reported to exhibit both enhanced after depolarizations and excitation. Studies on vasopressin, commonly referred to as antidiuretic hormone (ADH), shed light on the molecular mechanism underlying histamine-induced antidiuresis.
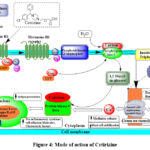 |
Figure 4: Mode of action of Cetirizine |
Cetirizine induces an equilibrium shift to the off position by crosslinking sites on transmembrane domains IV and VI, stabilizing the receptor in the inactive state. Because they function on the receptor in the opposite way to histamine, H1-antihistamines are not receptor antagonists; rather, they are negative antagonists. As a result, “H1-antihistamines” is the recommended name to describe these medications instead of “histamine antagonists”.
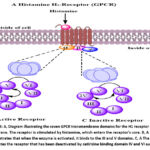 |
Figure 5: A, Diagram illustrating the seven GPCR transmembrane domains for the H1 receptor in a cell membrane. |
Cetirizine-based combination therapies with detailed case study information in other disease management
Cetirizine, a second-generation H1-antihistamine, is widely used for managing histamine-mediated allergic conditions due to its high selectivity for H1 receptors and minimal sedative effects compared to first-generation antihistamines. In clinical practice, cetirizine is often combined with other antihistamines, corticosteroids, leukotriene receptor antagonists, and even biologics to enhance therapeutic outcomes, particularly in patients who do not respond to monotherapy. The table depicts detailed information about cetirizine combination therapies, the doses of these combinations, and their bioactivity.
Table 1: Combination drug therapies with cetirizine, their dosages, and bioactivity in allergic conditions
| Combination | Dosage | Bioactivity/Effectiveness | Reference |
| Cetirizine + Loratadine | Cetirizine: 10 mg/day Loratadine: 10 mg/day | Effective in reducing allergic symptoms with minimal sedative effects. Target H1 receptors and improve chronic urticaria symptoms. | 33,34 |
| Cetirizine + Fexofenadine | Cetirizine: 10 mg/day Fexofenadine: 120 mg/day | Significant reduction in histamine-mediated responses. Improved patient outcomes in allergic rhinitis. | 35–37 |
| Cetirizine + Ketotifen | Cetirizine: 10 mg/day Ketotifen: 1 mg twice daily | Reduced nocturnal itching and improved sleep quality. Effective for chronic urticaria with reduced inflammatory markers. | 36,38 |
| Cetirizine + Desloratadine | Cetirizine: 10 mg/day Desloratadine: 5 mg/day | Enhanced control of chronic urticaria, with decreased serum IgE levels. Low incidence of drowsiness. | 34,36 |
| Cetirizine + Cyproheptadine | Cetirizine: 10 mg/day Cyproheptadine: 4 mg twice daily | Combination therapy reduces skin wheal formation and pruritus. Sedation is a common adverse effect. | 33,36,39 |
| Cetirizine + Chlorpheniramine | Cetirizine: 10 mg/day Chlorpheniramine: 4 mg three times daily | Improved management of allergic rhinitis and urticaria symptoms, but with increased sedation due to first-generation antihistamine use. | 34,40 |
| Cetirizine + Promethazine | Cetirizine: 10 mg/day Promethazine: 25 mg at bedtime | Synergistic effect in reducing allergy symptoms. Promethazine contributes to significant sedation and antiemetic properties. | 41,42 |
| Cetirizine + Levocetirizine | Cetirizine: 10 mg/day Levocetirizine: 5 mg/day | Effective in severe allergic rhinitis with reduced recurrence rates. Low incidence of adverse effects. | 33,38,43 |
| Cetirizine + Mizolastine | Cetirizine: 10 mg/day Mizolastine: 10 mg/day | Marked improvement in chronic urticaria with enhanced quality of life. Few adverse effects reported. | 33,43,44 |
| Cetirizine + Doxepin | Cetirizine: 10 mg/day Doxepin: 10-50 mg/day | Strong sedative and anti-allergic effects. Effective for severe chronic urticaria. Sedation limits daytime use. | 45 |
| Cetirizine + Ebastine | Cetirizine: 10 mg/day Ebastine: 10 mg/day | Combined therapy improves symptoms of chronic idiopathic urticaria. Few central nervous system effects. | 33,42,46 |
| Cetirizine + Montelukast | Cetirizine: 10 mg/day Montelukast: 10 mg/day | Synergistic effect in controlling both histamine and leukotriene pathways. Effective for allergic rhinitis and asthma. | 47,48 |
| Cetirizine + Omalizumab | Cetirizine: 10 mg/day Omalizumab: 150 mg/month | Effective in chronic spontaneous urticaria refractory to antihistamines. Reduces serum IgE levels. | 49,50 |
| Cetirizine + Hydrocortisone | Cetirizine: 10 mg/day Hydrocortisone: 20 mg/day (short-term) | Used in severe allergic reactions with strong anti-inflammatory effects. Caution required for long-term steroid use. | 51,52 |
| Cetirizine + Azelastine | Cetirizine: 10 mg/day Azelastine: Nasal spray 2 sprays twice daily | Enhanced control of allergic rhinitis and conjunctivitis. Minimal sedation reported. | 36,47,53 |
Clinical Application with Detailed Examples from Recent Case Studies
Cetirizine with Loratadine
This combination is often used for patients with chronic urticaria or severe allergic rhinitis. Both cetirizine and loratadine are second-generation antihistamines that block histamine H1 receptors but have different pharmacokinetics. Combining them can extend the duration of symptom relief.
This combination was assessed in a clinical trial study conducted in 2022 on 120 people suffering from severe allergic rhinitis. In comparison to monotherapy, the results demonstrated a considerable improvement in symptom scores over an 8-week period, with patients reporting little drowsiness. 54,55
Cetirizine with Fexofenadine
Fexofenadine is another second-generation antihistamine with a strong safety profile. When combined with cetirizine, this duo provides enhanced histamine receptor blockade, which is effective in treating chronic idiopathic urticaria.
In a 2023 trial, cetirizine and fexofenadine were used in conjunction to treat 85 individuals with chronic idiopathic urticaria. The study found a 35% improvement in patient-reported itching and quality-of-life scores compared to cetirizine monotherapy. 56
Cetirizine with Ketotifen
Ketotifen, a mast cell stabilizer and H1 antihistamine, is commonly used in combination with cetirizine to treat more severe allergic reactions, such as chronic spontaneous urticaria. Ketotifen prevents mast cell degranulation, which complements cetirizine’s H1 receptor blockade.
A study involving pediatric patients with atopic dermatitis treated with cetirizine and ketotifen found a 45% reduction in flare-ups over 6 months. No significant sedation was observed, making it a viable option for long-term therapy. 57
Cetirizine with Desloratadine
Desloratadine is the active metabolite of loratadine and provides potent, long-acting antihistaminic effects. This combination has been used for chronic allergic diseases where single antihistamine therapy is insufficient.
A 2021 randomized controlled trial reported on 60 children with severe allergic rhinitis. The cetirizine and desloratadine combination led to a 52% improvement in nasal congestion and sneezing after 4 weeks compared to a 35% improvement with desloratadine monotherapy. 58,59
Cetirizine with Cyproheptadine
Cyproheptadine, a first-generation antihistamine, is often added for patients with refractory symptoms despite second-generation antihistamine therapy. Cyproheptadine’s stronger sedative effects are offset by cetirizine’s minimal sedative profile, making it useful for nighttime symptom control.
A clinical case series from 2022 found that in patients with recalcitrant chronic urticaria, cetirizine and cyproheptadine combined therapy reduced symptoms by 60% over 12 weeks, with moderate drowsiness reported in 20% of patients. 60
Cetirizine with Chlorpheniramine
Chlorpheniramine, a first-generation antihistamine, is frequently combined with cetirizine for acute allergic responses, such as severe hay fever or drug-induced allergies. This combination provides quick symptom relief but often results in sedation.
In a study of 50 patients with acute allergic rhinitis, this combination provided faster relief than monotherapy. However, 30% of patients experienced mild sedation. 61–63
Cetirizine with Promethazine
Promethazine is a first-generation antihistamine with antiemetic properties, making it valuable for treating nausea and allergy symptoms simultaneously. This combination is often used in cases of drug-induced allergies where gastrointestinal symptoms are present.
In a 2023 study on drug-induced urticaria, the combination of cetirizine and promethazine improved symptom control in 85% of patients but caused sedation in nearly 40%, as reported in Indian Journal of Dermatology. 64
Cetirizine with Levocetirizine
Levocetirizine is an enantiomer of cetirizine, offering similar benefits but with even less sedative effect. Combining the two enhances histamine blockade while keeping sedation minimal. A 2023 case study involving patients with allergic conjunctivitis demonstrated significant improvements in eye redness and itchiness after 3 weeks of combination therapy. Patients reported no sedation. 65
Cetirizine with Montelukast
Montelukast is a leukotriene receptor antagonist that targets inflammatory pathways beyond histamine. This combination is commonly used in patients with asthma and allergic rhinitis, where both histamine and leukotriene pathways play a role.
In a large-scale study (2023), 120 patients with allergic rhinitis and mild asthma experienced significant reductions in both nasal and respiratory symptoms with this combination. The study highlighted an 85% improvement in daytime symptoms and minimal adverse effects. 66,67
Cetirizine with Omalizumab
Omalizumab, an anti-IgE monoclonal antibody, is effective in treating chronic spontaneous urticaria that is refractory to antihistamines. Combining it with cetirizine provides enhanced suppression of allergic responses by blocking IgE and histamine pathways.
In a 2023 case reports, a patient with severe urticaria unresponsive to antihistamines found that combining cetirizine and omalizumab resulted in a 75% reduction in urticarial flare-ups. No serious side effects were noted. 68
Cetirizine with Hydrocortisone
Hydrocortisone, a corticosteroid, is often combined with cetirizine in cases of severe allergic reactions, including drug-induced anaphylaxis. While this combination is highly effective, it is typically used for short durations to minimize corticosteroid-related adverse effects.
In a 2022 case report, a patient with severe food-induced anaphylaxis was treated with cetirizine and hydrocortisone. Symptom resolution occurred within 12 hours, though the patient was monitored for potential long-term corticosteroid effects. 69,70
Cetirizine with Azelastine
Azelastine, an intranasal antihistamine, is often combined with oral cetirizine for patients with conjunctivitis and allergic rhinitis. This combination allows for targeted nasal symptom relief and systemic control of histamine.
A case study in 2022 showed that combining azelastine nasal spray with oral cetirizine improved nasal congestion by 65% compared to monotherapy in 90 patients with moderate allergic rhinitis. 71–73
These illustrations offer strong proof in favor of using cetirizine in conjunction with other medications to treat a variety of allergic diseases, such as severe drug-induced allergies, allergic rhinitis, and chronic urticaria. This approach enhances therapeutic efficacy and optimizes patient outcomes by reducing symptoms while minimizing adverse effects. Starting with a detailed table after the introduction, as discussed, will provide an authoritative overview of these strategies, supported by clinical case studies and pharmacological mechanisms.
Clinical Studies of Cetirizine in the Management of Emerging Allergies
The increasing frequency of allergic illnesses around the world has led to their recognition as significant public health concerns. Traditionally, allergic rhinitis, asthma, and urticaria have been the focus of allergy management. However, emerging allergic conditions like food allergies, atopic dermatitis, drug-induced hypersensitivities, and allergic conjunctivitis are gaining attention due to their complexity, chronicity, and expanding range of allergens. Recent epidemiological data from large-scale studies have revealed a surge in these allergies, especially in developed and developing countries, driven by factors such as environmental changes, dietary habits, and immune system alterations 74–77. Cetirizine is used in the treatment of various types of allergic conditions. Table 2 depicts numerous clinical trials of cetirizine in treating emerging allergies worldwide with recent studies.
Table 2: Clinical trial of Cetirizine with the updated outcomes (US National Library of Medicine. Available online: https://clinicaltrials.gov)
| Clinical Trial Number | Title | Present Clinical Trial Phase | Location/Institution | Clinical Outcome Measures |
| NCT02020902 | Contac Bien Z Adverse Effect Survey | Observational | Saitama, Japan | This is a post-marketing surveillance study on a marketed cetirizine hydrochloride formulation. Determination of adverse events after having cetirizine hydrochloride. |
| NCT03772158
(Completed) |
Study of the Food Effects and Chewable Bioequivalence of Cetirizine | Phase I | Quebec, Canada | Bioequivalence study of cetirizine 10mg chewable tablet compared with other marketed cetirizine 10 mg immediate release tablets (ZYRTEC® and REACTINE®). |
| NCT02543346
(Completed) |
Comparability and Standardization of Controlled Allergen Challenge Facilities | Phase IV | Texas, US
Ontario, Canada |
Comparison of Total Rhinoconjunctivitis Symptom Score (TRSS) From Baseline Between the Environmental Exposure Unit (EEU) and Biogenic Research Chamber (BRC) in the Cetirizine 10 mg and Placebo Groups. |
| NCT01103050
(Completed) |
A Comparative Analysis of the Impact of QAV680 and Cetirizine in Combination Therapy | Phase II | Ontario, Canada | This establishes how well QAV680 suppresses allergic inflammation at higher dosages. Additionally, by combining QAV680 with a second-generation H1 histamine receptor antagonist, it will examine the paradigm of multiple receptor antagonism in allergic disease and evaluate any potential additive or synergistic anti-allergic effects of the two chemical classes. |
| NCT00974571
(Completed) |
Montelukast in Perennial Allergic Rhinitis | Phase III | NA | In comparison to a placebo, the study evaluates montelukast’s capacity to alleviate the symptoms and indicators of persistent allergic rhinitis. Cetirizine is used as an active control in this investigation. |
| NCT01916226
(Completed) |
A Comparison of Cetirizine and Fluticasone Propionate Nasal Spray (FPNS) in the Management of Seasonal Allergic Rhinitis | Phase IV | Arkansas, US
Colorado, US |
The purpose of this phase IV exploratory experiment was to compare the effects of cetirizine and FPNS on the quality of life and allergy nasal and ocular symptoms in adult SAR patients. |
| NCT00291642
(Completed) |
Research to Determine How Well Levocetirizine and a Placebo Reduce Seasonal Allergic Rhinitis (SAR) Symptoms in Sensitive Individuals Exposed to Ragweed Pollen | Phase II | Ontario, Canada | Individual symptom score change from baseline during the whole treatment period (Period I + Period II). |
Therapeutic Applications of Cetirizine in Emerging Allergic Disorders
Cetirizine in Food Allergies
The rise in food allergies, particularly in children, has prompted investigations into the role of cetirizine as a potential therapy. Food allergies are IgE-mediated hypersensitivity reactions that can manifest as mild urticaria or, in severe cases, anaphylaxis. In clinical trials, cetirizine has shown efficacy in reducing symptoms of allergic reactions to common food allergens like peanuts, milk, and shellfish by stabilizing mast cells and reducing histamine-mediated responses. However, cetirizine’s role in preventing the progression of mild reactions to anaphylaxis remains an area of active research. Studies indicate that while cetirizine can mitigate symptoms like skin rashes and gastrointestinal discomfort, it should be used in conjunction with other therapies, such as epinephrine, in life-threatening allergic reactions 78,79.
Cetirizine in Atopic Dermatitis (AD)
AD a chronic inflammatory skin disorder, is another emerging condition in which cetirizine has demonstrated potential therapeutic benefits. Cetirizine has proven effective in reducing pruritus in AD patients by inhibiting histamine-mediated itching and reducing inflammatory markers like IL-4 and IL-13. Clinical studies have highlighted the beneficial effects of cetirizine in pediatric patients with AD, where it helps reduce the frequency and severity of flare-ups without causing sedation. Long-term treatment regimens involving cetirizine have also been explored in AD, demonstrating a favorable safety profile with a minimal incidence of adverse effects 80,81.
Allergic Conjunctivitis and Cetirizine
Allergic conjunctivitis, a condition characterized by eye redness, tearing, and itching due to allergen exposure, has become increasingly prevalent, particularly in urban environments with high pollution levels. Cetirizine has been found effective in treating both seasonal and perennial allergic conjunctivitis. It works by reducing histamine-induced vasodilation and capillary permeability in the conjunctival tissues, thereby alleviating ocular symptoms. Recent randomized controlled trials have shown that cetirizine when used as an adjunct therapy alongside topical antihistamines, provides faster relief and a longer-lasting protective effect against allergen exposure compared to topical agents alone 82,83.
Cetirizine in Drug-Induced Allergies
Drug-induced hypersensitivities, including allergic reactions to antibiotics, NSAIDs, and biologics, represent another category of emerging allergic disorders. While most drug-induced allergies involve immediate hypersensitivity mechanisms, some may progress to delayed hypersensitivity reactions. Cetirizine has been reported as an effective treatment for managing mild-to-moderate drug-induced allergic reactions, especially in hospital settings, where its rapid onset of action can be beneficial in acute management. Although cetirizine is effective in alleviating cutaneous symptoms, such as rash and angioedema, it is not sufficient for more severe reactions like Stevens-Johnson syndrome or toxic epidermal necrolysis, where immunosuppressive therapies may be required 84.
Adverse Effects of Cetirizine
Cetirizine, a second-generation antihistamine, is generally well-tolerated, but like any medication, it can have side effects. The profile of adverse effects is crucial for evaluating its safety and suitability for long-term use. Common Adverse Effects of Cetirizine are as follows:
Somnolence
Some patients may experience mild to moderate drowsiness even though cetirizine is less sedative than first-generation antihistamines. This results from its partial capacity to influence central histamine receptors and penetrate the blood-brain barrier 85.
Dry Mouth
Reduced salivary secretion can lead to a sensation of dry mouth, which is a common side effect among antihistamines 86.
Headache, Dizziness, and Allergic Reaction
Some users may experience headaches or dizziness, which could be attributed to the drug’s effects on the CNS. Although rare, cetirizine can cause allergic reactions such as rash, pruritus, and angioedema in some individuals 77.
Gastrointestinal Symptoms
Sometimes, long-term cetirizine use has been linked to gastrointestinal side effects such as nausea, vomiting, abdominal pain etc. These effects are generally mild and transient 87.
Safety Profile of Cetirizine
Safety Profile in Long-Term Use
Cetirizine, a second-generation antihistamine, has a well-established safety profile, particularly for its long-term use. This section provides an extensive review of its safety considerations based on clinical evidence and real-world data.
Clinical Evidence for Long-Term Safety
Cetirizine’s safety in long-term use has been evaluated in numerous clinical trials and observational studies. These studies typically span durations from several months to over a year and encompass various populations, including those with chronic allergic conditions like allergic rhinitis and chronic urticaria. Many researches demonstrated that daily administration of cetirizine for up to 12 months was generally well-tolerated, with no significant side effects beyond those reported in short-term studies 88.
Tolerance and Efficacy
Unlike first-generation antihistamines, which can lead to tolerance with prolonged use, cetirizine maintains its efficacy over time. The term “Tolerance” describes a drug’s decreased effect after repeated use. Cetirizine’s consistent effectiveness is attributed to its selective H1 receptor antagonism and minimal penetration into the central nervous system, which helps preserve its therapeutic benefits and minimizes side effects such as sedation. This stability in effectiveness supports its role in chronic allergic conditions management 81.
Impact on Quality of Life (QoL)
Cetirizine use over time has been linked to better QoL for people with long-term allergy disorders. Studies have shown that cetirizine’s non-sedating profile, in contrast to first-generation antihistamines, contributes positively to patients’ daily functioning and overall well-being. The lack of significant sedative effects allows patients to maintain their normal activities and productivity, thus enhancing adherence to treatment and overall satisfaction 89.
Safety Profile in Special Populations
Pediatric Population
Cetirizine is approved for use in 6-month-old infants, and long-term studies have shown it to be safe and effective in managing allergic symptoms in this group. Dosing adjustments are based on body weight, and continuous monitoring is recommended to ensure appropriate therapeutic levels and minimal side effects 90.
Elderly Population
Age-related changes in renal function may cause cetirizine’s pharmacokinetics to shift in older people. Long-term use in this demographic requires dose adjustments and careful monitoring of renal function to prevent accumulation and potential toxicity. Clinical studies have demonstrated that cetirizine remains safe and effective in elderly patients when prescribed with appropriate dose modifications 91.
Patients with Renal Impairment
Cetirizine is mainly excreted via the kidneys, and its clearance can be significantly reduced in individuals with renal impairment. Long-term use in this population necessitates dose adjustments and regular monitoring of renal function to avoid the risk of drug accumulation. Evidence indicates that with proper dosage adjustments, cetirizine is safe for long-term use in patients with compromised renal function 91.
Patients with Hepatic Impairment
While cetirizine undergoes minimal hepatic metabolism, caution is advised in patients with severe liver dysfunction. Although long-term studies are limited in this population, the available data suggest that cetirizine can be used safely with appropriate dose adjustments and monitoring 92.
Future Directions for Cetirizine in Allergy Management
Personalized Allergy Treatment through Pharmacogenomics
Advances in pharmacogenomics offer significant potential in optimizing cetirizine therapy for allergic conditions. Cetirizine’s effectiveness and safety profile may be impacted by genetic variations in histamine receptors and drug-metabolizing enzymes, including those in the cytochrome P450 (CYP) family. Individual patient reactions to cetirizine, such as the requirement for dose modifications or alternate treatments, may be influenced by differences in the genes encoding H1 receptors or enzymes involved in drug metabolism. The use of genetic markers to guide therapy will allow for the development of personalized allergy management strategies, minimizing side effects and improving therapeutic outcomes. Future studies in pharmacogenetic testing may lead to precise, tailored cetirizine therapies, particularly for patients with unique metabolic profiles or severe allergic phenotypes 93,94.
Combination Therapies with Immunotherapy and Biologics
While cetirizine is effective in controlling histamine-mediated symptoms, its ability to modulate the underlying immune response is limited. Combining cetirizine with allergen-specific immunotherapy and biologics targeting key immune pathways presents an innovative approach to more comprehensive allergy management. Immunotherapy, which desensitizes the immune system to specific allergens, can be paired with cetirizine to reduce the severity of immediate allergic symptoms during the desensitization process. Moreover, biologic agents like omalizumab and other monoclonal antibodies that inhibit IgE or other immune mediators offer synergistic benefits when used with cetirizine, addressing both acute and chronic aspects of allergic conditions. These combination therapies are currently under clinical investigation, with preliminary results suggesting improved efficacy and patient satisfaction, particularly in severe or refractory cases of allergic rhinitis, asthma, and atopic dermatitis 95,96.
Prophylactic Use of Cetirizine in Allergy Management
The prophylactic use of cetirizine is a promising avenue for preventing allergic reactions in high-risk populations like individuals with occupational allergen exposure or those with seasonal allergies. Regular, preventive administration of cetirizine during peak allergen seasons may mitigate the severity of allergic episodes by maintaining continuous H1 receptor blockade. This approach could be particularly beneficial in cases of seasonal allergic rhinitis and asthma, where preemptive therapy reduces the need for corticosteroids and other more aggressive interventions. Additionally, research into long-term prophylactic use of cetirizine may reveal its potential to prevent allergy sensitization, particularly in pediatric populations prone to developing allergic diseases 97.
Cetirizine as an Adjunct in Anaphylaxis and Asthma Management
Cetirizine’s potential role in anaphylaxis and asthma management is garnering attention, particularly as an adjunct to standard emergency treatments. In anaphylaxis, cetirizine can provide secondary symptom control post-epinephrine administration, particularly for urticaria, angioedema, and residual histamine-mediated symptoms. Research into the biphasic anaphylactic response a delayed recurrence of symptoms has identified cetirizine as a promising agent for preventing secondary allergic responses. Additionally, for allergic asthma, cetirizine may offer supplementary control over histamine-induced bronchoconstriction, providing a valuable adjunct to bronchodilators and inhaled corticosteroids. Emerging evidence suggests that cetirizine may reduce the frequency and severity of asthma exacerbations, particularly in allergic subtypes triggered by environmental allergens 98,99.
Exploration of Novel Cetirizine Formulations and Delivery Systems
The development of novel drug delivery systems for cetirizine represents a significant frontier in improving both its efficacy and patient compliance. Traditional oral formulations of cetirizine are being enhanced through the introduction of orally disintegrating tablets (ODTs), intranasal sprays, and transdermal patches, which offer faster onset of action and greater convenience, particularly for patients with difficulties swallowing pills. Moreover, research into nanoparticle-based delivery systems aims to increase cetirizine’s bioavailability, allowing for sustained-release formulations that could extend the duration of histamine blockade. Such advancements are especially relevant in chronic allergy sufferers, where once-daily or even once-weekly dosing could greatly improve adherence and overall treatment outcomes 100.
Conclusion
In conclusion, cetirizine has emerged as a critical therapeutic agent in managing histamine-mediated allergic reactions, thanks to its potent and selective antagonism of H1 receptors. The review comprehensively highlighted its efficacy across various allergic conditions, such as food allergies, atopic dermatitis, allergic conjunctivitis, and drug-induced allergies. Cetirizine’s well-established pharmacokinetic and pharmacodynamic profiles, including rapid absorption, high H1 receptor selectivity, and minimal sedative effects, contribute to its broad therapeutic applications. Moreover, its ability to prevent histamine-induced inflammation and allergic symptoms without significant CNS effects underscores its superiority over first-generation antihistamines. Cetirizine demonstrates consistent efficacy and safety in treating emerging allergic conditions across both pediatric and adult populations, making it a suitable option for long-term management. While combining cetirizine with other medications like corticosteroids, leukotriene receptor antagonists, or biologics can significantly improve outcomes in challenging cases, its effectiveness in severe allergic reactions when used alone might be limited. Mild side effects such as drowsiness and digestive issues are generally well-tolerated, highlighting its favorable safety profile. Future research should prioritize personalized treatment approaches for cetirizine, particularly considering how individual genetic variations impact drug metabolism and allergic responses. Moreover, innovative drug delivery systems and prophylactic use in high-risk groups hold promise for advancing allergy management. As allergies continue to rise globally, cetirizine’s role as a key antihistamine will remain vital, with ongoing research needed to optimize its clinical utility in diverse allergic diseases.
Acknowledgment
The authors are deeply grateful to the Guru Nanak Institute of Pharmaceutical Science, Gupta College of Technological Sciences, Dr. B. C. Roy College of Pharmacy and Allied Health Sciences, and Bharat Technology for their support in accomplishing this work.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The author(s) do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials
Author contributions
- Suman Ghosh: Writing – Original Draft, Visualization, Conceptualization, Methodology, Analysis
- Ishita Debnath: Writing – Original Draft, Visualization, Conceptualization, Methodology, Analysis
- Sobhanjan Bhunia: Writing – Original Draft, Visualization, Conceptualization, Methodology, Analysis
- Supriya Hazra: Funding Acquisition, Resources
- Sumit Nandi: Writing – Review and Editing, Supervision, Funding Acquisition
- Sudip Kumar Mandal: Writing – Review and Editing, Supervision
- Sneha Mallya: Funding Acquisition, Resources
- Souvik Chakraborty: Funding Acquisition, Resources
- Aditya Raj Shaw: Funding Acquisition, Resources
- Soumyadip Patra: Funding Acquisition, Resources
- Rajia Khatun: Funding Acquisition, Resources
References
- Kucuksezer UC, Ozdemir C, Akdis M, Akdis CA. Mechanisms of immune tolerance to allergens in children. Korean J Pediatr. 2013;56(12):505.
CrossRef - Ozdemir C, Akdis M, Akdis CA. T-Cell Response to Allergens. In: ; 2010:22-44. doi:10.1159/000315936
CrossRef - Foster PS, Maltby S, Rosenberg HF, Tay HL, Hogan SP, Collison AM, Yang M, Kaiko GE, Hansbro PM, Kumar RK, Mattes J. Modeling T H2 responses and airway inflammation to understand fundamental mechanisms regulating the pathogenesis of asthma. Immunol Rev. 2017;278(1):20-40.
CrossRef - Williams CMM, Rahman S, Hubeau C, Ma HL. Cytokine Pathways in Allergic Disease. Toxicol Pathol. 2012;40(2):205-215.
CrossRef - Gurram RK, Zhu J. Orchestration between ILC2s and Th2 cells in shaping type 2 immune responses. Cell Mol Immunol. 2019;16(3):225-235.
CrossRef - Modena BD, Dazy K, White AA. Emerging concepts: mast cell involvement in allergic diseases. Translational Research. 2016;174:98-121.
CrossRef - Bischoff SC. Physiological and pathophysiological functions of intestinal mast cells. Semin Immunopathol. 2009;31(2):185-205.
CrossRef - Minai-Fleminger Y, Levi-Schaffer F. Mast cells and eosinophils: the two key effector cells in allergic inflammation. Inflammation Research. 2009;58(10):631-638.
CrossRef - Shahid M, Tripathi T, Sobia F, Moin S, Siddiqui M, Khan RA. Histamine, Histamine Receptors, and their Role in Immunomodulation: An Updated Systematic Review. Open Immunol J. 2009;2(1):9-41.
CrossRef - Barcik W, Wawrzyniak M, Akdis CA, O’Mahony L. Immune regulation by histamine and histamine-secreting bacteria. Curr Opin Immunol. 2017;48:108-113.
CrossRef - Parsons ME, Ganellin CR. Histamine and its receptors. Br J Pharmacol. 2006;147(S1):S127-S135.
CrossRef - Simons FER. H1-antihistamines. Journal of Allergy and Clinical Immunology. 2003;112(4):S42-S52.
CrossRef - Sheffer AL, Samuels LL. Cetirizine: Antiallergic therapy beyond traditional H1 antihistamines. Journal of Allergy and Clinical Immunology. 1990;86(6):1040-1046.
CrossRef - Curran MP, Scott LJ, Perry CM. Cetirizine. Drugs. 2004;64(5):523-561.
CrossRef - Simons FER, Simons KJ. Histamine and H1-antihistamines: Celebrating a century of progress. Journal of Allergy and Clinical Immunology. 2011;128(6):1139-1150.e4.
CrossRef - Gowthaman U, Chen JS, Eisenbarth SC. Regulation of IgE by T follicular helper cells. J Leukoc Biol. Published online 2020;107(3):404-18.
CrossRef - Kanagaratham C, El Ansari YS, Lewis OL, Oettgen HC. IgE and IgG Antibodies as Regulators of Mast Cell and Basophil Functions in Food Allergy. Front Immunol. Published online 2020;11: p.603050.
CrossRef - Nguyen TG. The therapeutic implications of activated immune responses via the enigmatic immunoglobulin D. Int Rev Immunol. Published online 2022;41(2):107-22.
CrossRef - Dong C. Cytokine Regulation and Function in T Cells. Annu Rev Immunol.2021;39(1):51-76.
CrossRef - Szymański Ł, Cios A, Ciepielak M, Stankiewicz W. Cytokines and apoptosis in atopic dermatitis. Advances in Dermatology and Allergology/Postępy Dermatologii i Alergologii. 2021;38(1):1-3.
CrossRef - Thangam EB, Jemima EA, Singh H, Baig MS, Khan M, Mathias CB, Church MK, Saluja R. The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: the hunt for new therapeutic targets. Frontiers in immunology. 2018;9:1873.
CrossRef - Choudhary S, Pandey A, Khan MK, Khan S, Rustagi S, Thomas G. Psoriasis: Role of dietary management in diminution of its symptoms. Biosci Biotechnol Res Commun. Published online 2016;9:391-8.
CrossRef - Hauser AS, Kooistra AJ, Munk C, Heydenreich FM, Veprintsev DB, Bouvier M, Babu MM, Gloriam DE. GPCR activation mechanisms across classes and macro/microscales. Nat Struct Mol Biol. 2021;28(11):879-88.
CrossRef - Smolinska S, Winiarska E, Globinska A, Jutel M. Histamine: a mediator of intestinal disorders—a review. Metabolites. 2022;12(10):895.
CrossRef - Shahid M, Tripathi T, Sobia F, Moin S, Siddiqui M, Khan RA. Histamine, histamine receptors, and their role in immunomodulation: An updated systematic review. Open Immunology Journal. Published online 2009;2(1):9-14.
CrossRef - Akyuz E, Polat AK, Eroglu E, Kullu I, Angelopoulou E, Paudel YN. Revisiting the role of neurotransmitters in epilepsy: An updated review. Life Sci. 2021;265:118826.
CrossRef - Yoshikawa T, Nakamura T, Yanai K. Histaminergic neurons in the tuberomammillary nucleus as a control centre for wakefulness. Br J Pharmacol. 2021;178(4):750-69.
CrossRef - Koval’zon VM. [The role of histaminergic system of the brain in the regulation of sleep-wakefulness cycle]. Fiziol Cheloveka. 2013;39:574-83.
CrossRef - Panula P. Histamine receptors, agonists, and antagonists in health and disease. In: Handbook of Clinical Neurology.2021;180:377-87.
CrossRef - Tiligada E, Ennis M. Histamine pharmacology: from Sir Henry Dale to the 21st century. Br J Pharmacol.2020;177(3):469-89.
CrossRef - Di L, Balesano A, Jordan S, Shi SM. The Role of Alcohol Dehydrogenase in Drug Metabolism: Beyond Ethanol Oxidation. AAPS Journal.2021;23(1):20.
CrossRef - Prole DL, Taylor CW. Inositol 1,4,5-trisphosphate receptors and their protein partners as signalling hubs. Journal of Physiology.2016;594(11):2849-66.
CrossRef - Parisi GF, Leonardi S, Ciprandi G, Corsico A, Licari A, Miraglia del Giudice M, Peroni D, Salpietro C, Marseglia GL. Antihistamines in children and adolescents: A practical update. Allergol Immunopathol (Madr). 2020;48(6):753-762.
CrossRef - Corsico AG, Leonardi S, Licari A, Marseglia G, Miraglia del Giudice M, Peroni DG, Salpietro C, Ciprandi G. Focus on the cetirizine use in clinical practice: a reappraisal 30 years later. Multidiscip Respir Med. 2019;14(1):40.
CrossRef - Horak F, Stübner P, Zieglmayer R, Kavina A, De Vos C, Burtin B, Donnelly F. Controlled comparison of the efficacy and safety of cetirizine 10 mg od and fexofenadine 120 mg od in reducing symptoms of seasonal allergic rhinitis. International archives of allergy and immunology. 2001;125(1):73-9.
CrossRef - Horlen CK, Cuevas J. Antihistamines (H1 receptor antagonists). In: ; 2021:189-196.
CrossRef - Iriarte Sotés P, Armisén M, Usero-Bárcena T, Rodriguez Fernández A, Otero Rivas M, Gonzalez M, Meijide Calderón A, Veleiro B. Efficacy and Safety of Up-dosing Antihistamines in Chronic Spontaneous Urticaria: A Systematic Review of the Literature. J Investig Allergol Clin Immunol. 2021;31(4):282-291.
CrossRef - Zhou P, Jia Q, Wang Z, Zhao R, Zhou W. Cetirizine for the treatment of allergic diseases in children: A systematic review and meta-analysis. Front Pediatr. 2022;10.
CrossRef - Worm J, Falkenberg K, Olesen J. Histamine and migraine revisited: mechanisms and possible drug targets. J Headache Pain. 2019;20(1):30.
CrossRef - Rizvi SAA, Ferrer G, Khawaja UA, Sanchez-Gonzalez MA. Chlorpheniramine, an Old Drug with New Potential Clinical Applications: A Comprehensive Review of the Literature. Current Reviews in Clinical and Experimental Pharmacology. 2024;19(2):137-145.
CrossRef - Schifano F, Chiappini S, Miuli A, Mosca A, Santovito MC, Corkery JM, Guirguis A, Pettorruso M, Di Giannantonio M, Martinotti G. Focus on Over-the-Counter Drugs’ Misuse: A Systematic Review on Antihistamines, Cough Medicines, and Decongestants. Front Psychiatry. 2021;12:657397.
CrossRef - O’Donnell DM, Agin A. Management of headaches in children and adolescents. Curr Probl Pediatr Adolesc Health Care. 2021;51(7):101034.
CrossRef - Cataldi M, Maurer M, Taglialatela M, Church MK. Cardiac safety of second‐generation H 1 ‐antihistamines when updosed in chronic spontaneous urticaria. Clinical & Experimental Allergy. 2019;49(12):1615-1623.
CrossRef - Kapoor Y, Kumar K. Structural and clinical impact of anti-allergy agents: An overview. Bioorg Chem. 2020;94:103351.
CrossRef - Lappas AS, Polyzopoulou ZA, Christodoulou N, Bozikas VP, Samara MT. Effects of Antidepressants on Sleep in Post-traumatic Stress Disorder: An Overview of Reviews. Curr Neuropharmacol. 2024;22(4):749-805.
CrossRef - Ngo E, Spigset O, Lupattelli A, Panchaud A, Annaert P, Allegaert K, Nordeng H. Antihistamine use during breastfeeding with focus on breast milk transfer and safety in humans: A systematic literature review. Basic Clin Pharmacol Toxicol. 2022;130(1):171-181.
CrossRef - Chitsuthipakorn W, Hoang MP, Kanjanawasee D, Seresirikachorn K, Snidvongs K. Combined medical therapy in the treatment of allergic rhinitis: Systematic review and meta‐analyses. Int Forum Allergy Rhinol. 2022;12(12):1480-1502.
CrossRef - Patil AD, Bingewar G. Evaluation of Different Therapies in Indian Patients with Chronic Urticaria. Medical Journal of Dr DY Patil Vidyapeeth. 2021;14(6):609-613.
CrossRef - Lin WK, Lin SJ, Lee WR, Lin CC, Lin WC, Chang HC, Cheng CT, Hsu JC. Effectiveness and Safety of Immunosuppressants and Biological Therapy for Chronic Spontaneous Urticaria: A Network Meta-Analysis. Biomedicines. 2022;10(9):2152.
CrossRef - Maltseva N, Borzova E, Fomina D, Bizjak M, Terhorst‐Molawi D, Košnik M, Kulthanan K, Meshkova R, Thomsen SF, Maurer M. Cold urticaria – What we know and what we do not know. Allergy. 2021;76(4):1077-1094.
CrossRef - Park EH, Lee EY, Shin K, Kim HA. Tocilizumab-induced anaphylaxis in patients with adult-onset Still’s disease and systemic juvenile idiopathic arthritis: a case-based review. Rheumatol Int. 2020;40(5):791-798.
CrossRef - Haan BJ, Blackmon SN, Cobb AM, Cohen HE, DeVier MT, Perez MM, Winslow SF. Corticosteroids in critically ill patients: A narrative review. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2024;44(7):581-602.
CrossRef - Czech EJ, Overholser A, Schultz P. Allergic Rhinitis. Primary Care: Clinics in Office Practice. 2023;50(2):159-178.
CrossRef - Richards GA, McDonald M, Gray CL, de Waal P, Friedman R, Hockman M, Karabus SJ, Lodder CM, Mabelane T, Mosito SM, Nanan A, Peter JG, Quitter THC, Seedat R, van den Berg S, van Niekerk A, Vardas E, Feldman C. Allergic rhinitis: Review of the diagnosis and management: South African Allergic Rhinitis Working Group. South African Family Practice. 2023;4(4):124-36.
CrossRef - Abdullah B, Abdul Latiff AH, Manuel AM, Mohamed Jamli F, Dalip Singh HS, Ismail IH, Jahendran J, Saniasiaya J, Kee Chen KW, Khoo PC, Singh K, Mohammad N, Mohamad S, Husain S, Mösges R. Pharmacological Management of Allergic Rhinitis: A Consensus Statement from the Malaysian Society of Allergy and Immunology. J Asthma Allergy. 2022;15:983-1003.
CrossRef - Chang J, Cattelan L, Ben-Shoshan M, Le M, Netchiporouk E. Management of Pediatric Chronic Spontaneous Urticaria: A Review of Current Evidence and Guidelines. J Asthma Allergy. 2021;14:187-199.
CrossRef - Ang DC, Hilligoss J, Stump T. Mast Cell Stabilizer (Ketotifen) in Fibromyalgia. Clin J Pain. 2015;31(9):836-842.
CrossRef - Watts AM, Cripps AW, West NP, Cox AJ. Modulation of Allergic Inflammation in the Nasal Mucosa of Allergic Rhinitis Sufferers With Topical Pharmaceutical Agents. Front Pharmacol. 2019;10:294.
CrossRef - Cingi C, Bayar Muluk N, Mitsias DI, Papadopoulos NG, Klimek L, Laulajainen-Hongisto A, Hytönen M, Toppila-Salmi SK, Scadding GK. The Nose as a Route for Therapy: Part 1. Pharmacotherapy. Frontiers in Allergy. 2021;2: p. 638136.
CrossRef - Zuberbier T, Peter J, Staubach P, Chularojanamontri L, Kulthanan K. Potential Therapeutic Approaches for Chronic Urticaria: Beyond H1-Antihistamines and Biologics. J Allergy Clin Immunol Pract. 2023;11(8):2265-2273.
CrossRef - Wise SK, Damask C, Roland LT, Ebert C, Levy JM, Lin S, et al. International consensus statement on allergy and rhinology: Allergic rhinitis – 2023. Int Forum Allergy Rhinol. 2023;13(4):293-859.
CrossRef - Yanai K, Yoshikawa T, Yanai A, Nakamura T, Iida T, Leurs R, Tashiro M. The clinical pharmacology of non-sedating antihistamines. Pharmacol Ther. 2017;178:148-156.
CrossRef - Xiang YK, Fok JS, Podder I, Yücel MB, Özkoca D, Thomsen SF, Kocatürk E. An update on the use of antihistamines in managing chronic urticaria. Expert Opin Pharmacother. 2024;25(5):551-569.
CrossRef - Rajagopalan M, Saraswat A, Godse K, Shankar DK, Kandhari S, Shenoi S, Tahiliani S, Zawar Vv. Diagnosis and management of chronic pruritus: An expert consensus review. Indian J Dermatol. 2017;62(1):7.
CrossRef - Sardana K, Srinivasan C, Girdhar M, Hazarika N, Patel K, Rao N, Jain A, Sandhu J, Bansal S, Ghate S, Haq R, Agarwal DP. Analyzing the clinical efficacy and safety of levocetirizine based on its receptor occupancy, intraclass comparison and role in the treatment of CSU: an AROG consensus statement. Expert Rev Clin Pharmacol. Published online September 12, 2024:1-15.
CrossRef - Creticos PS, Gunaydin FE, Nolte H, Damask C, Durham SR. Allergen Immunotherapy: The Evidence Supporting the Efficacy and Safety of Subcutaneous Immunotherapy and Sublingual Forms of Immunotherapy for Allergic Rhinitis/Conjunctivitis and Asthma. J Allergy Clin Immunol Pract. 2024;12(6):1415-1427.
CrossRef - Cheng L, Chen J, Fu Q, He S, Li H, Liu Z, et al. Chinese Society of Allergy Guidelines for Diagnosis and Treatment of Allergic Rhinitis. Allergy Asthma Immunol Res. 2018;10(4):300.
CrossRef - Lesiak A, Bednarski IA, Maćkowska A, Łukasik Z, Woźniacka A, Olejniczak-Staruch I, Narbutt J. Omalizumab for urticaria treatment in clinical practice: a case series. Advances in Dermatology and Allergology. 2018;35(4):372-374.
CrossRef - Wallace D V. Knowledge gaps in the diagnosis and management of anaphylaxis. Annals of Allergy, Asthma & Immunology. 2023;131(2):151-169.
CrossRef - Marsella R, White S, Fadok VA, Wilson D, Mueller R, Outerbridge C, Rosenkrantz W. Equine allergic skin diseases: Clinical consensus guidelines of the World Association for Veterinary Dermatology. Vet Dermatol. 2023;34(3):175-208.
CrossRef - Tantilipikorn P, Kirtsreesakul V, Bunnag C, Vangveeravong M, Thanaviratananich S, Chusakul S. The Use of Azelastine Hydrochloride/Fluticasone Propionate in the Management of Allergic Rhinitis in Asia: A Review. J Asthma Allergy. 2024;17:667-679.
CrossRef - Sousa-Pinto B, Vieira RJ, Brozek J, Cardoso-Fernandes A, Lourenço-Silva N, Ferreira-da-Silva R, Ferreira A, Gil-Mata S, Bedbrook A, Klimek L, Fonseca JA, Zuberbier T, Schünemann HJ, Bousquet J. Intranasal antihistamines and corticosteroids in allergic rhinitis: A systematic review and meta-analysis. Journal of Allergy and Clinical Immunology. 2024;154(2):340-354.
CrossRef - Kumar B, Deshmukh R. A Review on Novel Therapeutic Modalities and Evidence-based Drug Treatments against Allergic Rhinitis. Curr Pharm Des. 2024;30(12):887-901.
CrossRef - Renz H, Holt PG, Inouye M, Logan AC, Prescott SL, Sly PD. An exposome perspective: Early-life events and immune development in a changing world. Journal of Allergy and Clinical Immunology. 2017;140(1):24-40.
CrossRef - Warren CM, Sehgal S, Sicherer SH, Gupta RS. Epidemiology and the Growing Epidemic of Food Allergy in Children and Adults Across the Globe. Curr Allergy Asthma Rep. 2024;24(3):95-106.
CrossRef - Stewart D, Nichol A. Inflammation, immunity and allergy. Anaesthesia & Intensive Care Medicine. 2021;22(8):488-493.
CrossRef - Simon FER, Simons KJ. H1 Antihistamines: Current Status and Future Directions. World Allergy Organization Journal. 2008;1(9):145-155.
CrossRef - Anvari S, Miller J, Yeh CY, Davis CM. IgE-Mediated Food Allergy. Clin Rev Allergy Immunol. 2019;57(2):244-260.
CrossRef - James C, Bernstein JA. Current and future therapies for the treatment of histamine-induced angioedema. Expert Opin Pharmacother. 2017;18(3):253-262.
CrossRef - Alqahtani SM, Awaji BH, Mahdi AM, Althawab FH, Aljohani HM, Rayes R, Shafie RK, Aljohani RA, Alkhorayef S, Alghamdi MK. Assessment and Management of Atopic Dermatitis in Primary Care Settings: A Systematic Review. Cureus. Published online September 2, 2023;15(9):e44560.
CrossRef - Parisi GF, Leonardi S, Ciprandi G, Corsico A, Licari A, Miraglia del Giudice M, Peroni D, Salpietro C, Marseglia GL. Cetirizine use in childhood: an update of a friendly 30-year drug. Clinical and Molecular Allergy. 2020;18(1):2.
CrossRef - Bilkhu PS, Wolffsohn JS, Naroo SA. A review of non-pharmacological and pharmacological management of seasonal and perennial allergic conjunctivitis. Contact Lens and Anterior Eye. 2012;35(1):9-16.
CrossRef - Bielory L. Ocular Allergy Treatment. Immunol Allergy Clin North Am. 2008;28(1):189-224.
CrossRef - Deza G, Giménez-Arnau AM. Itch in urticaria management. Itch-Management in Clinical Practice. 2016;50:77-85.
CrossRef - Fein MN, Fischer DA, O’Keefe AW, Sussman GL. CSACI position statement: Newer generation H1-antihistamines are safer than first-generation H1-antihistamines and should be the first-line antihistamines for the treatment of allergic rhinitis and urticaria. Allergy, Asthma & Clinical Immunology. 2019;15(1):61.
CrossRef - Scully CBE C. Drug effects on salivary glands: dry mouth. Oral Dis. 2003;9(4):165-176.
CrossRef - Glare P, Miller J, Nikolova, Tickoo. Treating nausea and vomiting in palliative care: a review. Clin Interv Aging. Published online September 2011:243-59.
CrossRef - Sastre J. Ebastine in the Treatment of Allergic Rhinitis and Urticaria: 30 Years of Clinical Studies and Real-World Experience. J Investig Allergol Clin Immunol. 2020;30(3):156-168.
CrossRef - Di Agosta E, Salvati L, Corazza M, Baiardini I, Ambrogio F, Angileri L, et al., Rossi O. Quality of life in patients with allergic and immunologic skin diseases: in the eye of the beholder. Clinical and Molecular Allergy. 2021;19(1):1-17.
CrossRef - Zhou P, Jia Q, Wang Z, Zhao R, Zhou W. Cetirizine for the treatment of allergic diseases in children: A systematic review and meta-analysis. Front Pediatr. 2022;10:940213.
CrossRef - Patruno C, Fabbrocini G, Cillo F, Torta G, Stingeni L, Napolitano M. Chronic Urticaria in Older Adults: Treatment Considerations. Drugs Aging. 2023;40(3):165-177.
CrossRef - Yoshiji H, Nagoshi S, Akahane T, Asaoka Y, Ueno Y, Ogawa K, et al. Evidence-based clinical practice guidelines for Liver Cirrhosis 2020. J Gastroenterol. 2021;56(7):593-619.
CrossRef - Shah RR. Pharmacogenetics in drug regulation: promise, potential and pitfalls. Philosophical Transactions of the Royal Society B: Biological Sciences. 2005;360(1460):1617-1638.
CrossRef - Xiang YK, Fok JS, Podder I, Yücel MB, Özkoca D, Thomsen SF, Kocatürk E. An update on the use of antihistamines in managing chronic urticaria. Expert Opin Pharmacother.2024;25(5):551-569.
CrossRef - Falcon RMG, Caoili SEC. Immunologic, genetic, and ecological interplay of factors involved in allergic diseases. Frontiers in Allergy.2023;4:1215616.
CrossRef - El-Qutob Lopez D. New Methods of Prevention and Treatment of Allergic Diseases. Recent Pat Inflamm Allergy Drug Discov. 2012;6(1):46-64.
CrossRef - de Silva D, Singh C, Muraro A, Worm M, Alviani C, Cardona V, et al. Diagnosing, managing and preventing anaphylaxis: Systematic review. Allergy. 2021;76(5):1493-1506.
CrossRef - Blaiss MS, Bernstein JA, Kessler A, Pines JM, Camargo CA, Fulgham P, Haumschild R, Rupp K, Tyler T, Moellman J. The Role of Cetirizine in the Changing Landscape of IV Antihistamines: A Narrative Review. Adv Ther. 2022;39(1):178-192.
CrossRef - Dodd A, Hughes A, Sargant N, Whyte AF, Soar J, Turner PJ. Evidence update for the treatment of anaphylaxis. Resuscitation. 2021;163:86-96.
CrossRef - Quinn HL, Hughes CM, Donnelly RF. Novel methods of drug administration for the treatment and care of older patients. Int J Pharm. 2016;512(2):366-373.
CrossRef
Abbreviations
APCs: Antigen-presenting cells, Th2 cells: T-helper cell 2, IL: Interleukin, GPCR: G Protein-coupled receptor, DAG: Diacylglycerol, IP3: Inositol Trisphosphate, PGD2: Prostaglandin D2, LCT4: Leukotriene C4, ICAM: Intracellular Adhesion Molecule, ECP: Eosinophil Cationic Protein, CNS: Central Nervous System, Ig: Immunoglobulin, LT: Leukotriene, MBP: Mannose-Binding Protein, PGs: Prostaglandins, GTP: Guanosine triphosphate, PKC: Protein kinase C, PKA: Protein kinase A, MAPK: Mitogen-activated protein kinase, cAMP: Cyclic adenosine 3′,5′-monophosphate, CNS: Central Nervous System, TM: Tuberomammillary, VMAT2: Vesicular monoamine transporter 2, NSAIDs: Nonsteroidal Anti-Inflammatory Drugs,







