Yogita Ale1 , Rekha Verma2
, Rekha Verma2 , Nidhi Nainwal1
, Nidhi Nainwal1 , Sanjeev Kumar Shah3
, Sanjeev Kumar Shah3 , Vikash Jakhmola4
, Vikash Jakhmola4 , Pankaj Pant5
, Pankaj Pant5
1Department of Pharmaceutics, Uttaranchal Institute of Pharmaceutical Sciences, Uttaranchal University, Dehradun, Uttarakhand, India.
2Department of Management, DBS Global University, Dehradun, Uttarakhand, India.
3Uttaranchal Institute of Technology, Uttaranchal University, Dehradun-248007, Uttarakhand, India.
4Department of Pharmaceutical Chemistry, Uttaranchal Institute of Pharmaceutical Sciences, Uttaranchal University, Dehradun-248007, Uttarakhand, India.
5Faculty of Pharmacy, School of Pharmaceutical and Population Health Informatics, DIT University, Dehradun-248009, Uttarakhand, India.
Corresponding Author E-mail:yogitaale7@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/3069
Abstract
The enactment of advanced technologies and processes accomplished by the Internet of Things (IoT), Artificial Intelligence (AI), Cloud Computing, and Big Data for development over domestic and global markets represent the new paradigm, Industry 4.0. The application of these technologies in the pharmaceutical industry and other health sector will simplify the production of complex medications, which is a major benefit of the paradigm shift. Industry 4.0 was implemented to streamline complex processes and reduce reliance on human labour. Although most pharmaceutical industries still understand the concept of Industry 3.0, it is challenging for manufacturers and regulators to cope with the current scenario and meet the guidelines and requirements of different regulatory bodies. This review article highlighted the application of IoT and other novel advanced technologies in the pharmaceutical sector. Additionally, outlined the major challenges including high-cost installations, the need for expertise and training, and regulatory enforcement. Industries and regulatory bodies are working together to develop computational engineering methodologies necessary to support and promote autonomous systems with artificial intelligence and computing infrastructures for the development of the pharmaceutical sector.
Keywords
Artificial Intelligence; Cloud computing; Digitalization; Industrial revolutions; Internet of Things
Download this article as:| Copy the following to cite this article: Ale Y, Verma R, Nainwal N. Jakhmola V, Pant P. IoT in Revolutionizing the Pharmaceutical Sector: Applications and Challenges. Biomed Pharmacol J 2025;18(March Spl Edition). |
| Copy the following to cite this URL: Ale Y, Verma R, Nainwal N. Jakhmola V, Pant P. IoT in Revolutionizing the Pharmaceutical Sector: Applications and Challenges. Biomed Pharmacol J 2025;18(March Spl Edition). Available from: https://bit.ly/3Dbnm2e |
Introduction
Industry 4.0 refers to the fourth industrial revolution, characterized by the integration of digital technologies, the Internet of Things (IoT), artificial intelligence (AI), big data, and other advanced technologies into the manufacturing and industrial processes. This concept represents a significant shift from traditional industrial practices to smart manufacturing systems that are more connected, automated, and data-driven. The goal of Industry 4.0 is to create “smart factories” that are more efficient, flexible, and responsive to market demands. It transforms traditional manufacturing into a more interconnected and intelligent ecosystem, ultimately leading to improved productivity, reduced costs, and enhanced competitiveness for industries. The term “Industry 4.0” originated in Germany and is part of the broader framework for the future of manufacturing and industrial processes1,2.
Industry 4.0 might help the pharmaceutical sector with enhanced manufacturing, additive manufacturing, personalized medicine, localized 3D printing of therapies, and even a time when production is done without direct human involvement. This future is attainable if industry and educational institutions work together to promote progressive research and implementation of Industry 4.0. The proliferation of cyber-physical system design paradigms has substantially broadened the range of interoperable systems that will be required in future enterprises. Industry 4.0 encompasses all facets associated with an industrial operational paradigm, such as regulatory interaction, management accountability, and organizational culture. The manufacturing sector has undergone a transformation from mass production to mass customization, which has sparked industry-wide Industry 4.0–related developments including end-to-end supply chain integration, personalized treatments, and data integrity by design. The goal of Industry 4.0 is to create smart factories by reimagining the place of humans in the manufacturing process through the productive collaboration of cyber and physical systems3,4. The nascent technologies of Industry 4.0 enable the generation of sustainable value and contribute to a pharmaceutical sector that is more adaptable, intelligent, and individualized; thus, they provide pharmaceutical firms a competitive edge. It is imperative to establish a pharmaceutical supply chain that is more sustainable in order to align with the management and future operations of pharmaceutical products throughout their complete life cycle. Pharma 4.0 is a concept that aims to transform the pharmaceutical industry through the integration of digital technologies into every aspect of drug development and manufacturing. It builds on the principles of Industry 4.0, which refers to the fourth industrial revolution characterized by the use of cyber-physical systems, the internet of things (IoT), and cloud computing in manufacturing. Pharma 4.0 aims to improve the efficiency and effectiveness of drug development, reduce costs, and improve patient outcomes. These technologies could potentially enhance efficiency, quality, and traceability in the pharmaceutical industry4.
Currently, many pharmaceutical industries are trying to implement industry 4.0 concept but also facing strict regulations by several regulators and stake holders for quality, safety and efficacy of pharmaceutical product. Since many years pharmaceutical industries are still in Industry 2.0 manufacturing batch-based production due to robust on-line quality control and flexible production which result in shortage of products even when emergency situations occur. Therefore, it is necessary to improve the accessibility of medicines by pharmaceutical industries. Emerging technologies of Industry 4.0 like real-time data processing, 3D printing, AI and robots are they key features of on-line quality controls and close-loops of continuous manufacturing production5. Beside from pharmaceutical manufacturing, other departments of industries like logistics, supply chain distributors also need to implement advanced technologies such as auto ID tags, patient centric information exchange, cloud computing, big data analytics, to deal with future demands of Pharma 4.0. While employing novel technologies of Industry 4.0 may be challenging but it will be worthy as it has potential to provide higher manufacturing outputs and safety, increase product quality, flexibility and reduced waste 6. Figure 1. represents the revolution of the pharmaceutical industry, highlighting the key elements of each stage from the manual process of manufacturing medicines from medicinal plants to the cutting-edge manufacturing technologies.
Industrial Revolutions in Pharmaceutical Industry
Industry 1.0
Herbal or botanical substances have been employed as therapeutic agents throughout the history of civilization. The manual processing of substances derived from plants, minerals, and animals progressed from manual tools to massive machines capable of crushing, grinding, combining, and pressing more medications6. Individual pharmacies and industries of chemical or dyes—began making medications on a larger scale in the 19th century using machinery powered by sources other than electricity. Few early innovations from the industry 1.0 revolution, are pneumatic mills and tablet presses which are still used in industries. The first industrial revolution began in the late 18th century and was characterized by the use of mechanization to replace manual labour. The introduction of steam power and the development of textile mills were significant innovations during this era7.
Industry 2.0
This revolution was made possible by electricity, the invention of electronic technology. Production process with pre-programmed controls and critical point process controls that allowed companies to specify basic process parameters. Machines operating from electricity such as crushing, milling, mixing, and tablet pressing, enables larger-scale production and critically greater process and access improved quality control. Process controls however, were often bound to static, fixed parameters that only permitted for passive control methods and the monitoring of process performance. Invention of electronic tablet presses that can consistently give output of more than a million tablets per hour. The second industrial revolution started in the late 19th century and was driven by the introduction of electricity, the assembly line, and mass production techniques. These developments allowed for faster and more efficient manufacturing processes7,8.
Industry 3.0
This revolution was aided by the incorporation and accessibility of computer and communication technologies including networked computers, internet, and wireless system. Continuous manufacturing and active control were made possible by these technologies’ greater ability to automate machinery and procedures. The development of more complicated control techniques and an improvement in process and product quality were both facilitated by human-computer interfaces. Lowering the requirement for manual operators in the factory, remote sensing and monitoring enabled monitoring and tracking of production-related indications8. Advanced Process analytical technology (PAT), was introduced to the pharmaceutical manufacturing industry for providing quality data of products and manufacturing process. Incorporation of advanced Quality by Design (QbD) procedures was made to control and monitor desired product quality profiles under predetermined range of quality criteria. The third industrial revolution began in the late 20th century and was characterized by the introduction of automation and the use of computers in manufacturing. This allowed for more precise and accurate production processes, as well as increased efficiency and reduced costs8.
Industry 4.0
This revolution’s is about the adoption of cutting-edge manufacturing technology paves the way for interconnected, automatic, robotic and self-organized production systems that function without the need for human oversight. Algorithms can be used in this situation to analyse performance data and make critical real-time operational business decision impacting production outputs. From simple data to digital maturity, data must be transformed from raw manufacturing process to information received through evaluation of data, knowledge created through the addition of contextual meaning, possibly by artificial intelligence, and finally, actionable wisdom to support decision-making by the contribution of insight. Autonomous systems and cyber-physical devices are propelled by this “intelligence,” which includes computer-controlled mechanisms and enables them to self-optimize, make choices, move remotely, and use adaptive control1,8. New operational paradigms brought about by automation, digitalization and real-time integration may make it possible for pharmaceutical manufacturing to attain more than six sigma quality for both small- and large-scale pharmaceutical manufacture. During COVID-19 emergency, highlighted the time to change the manufacturing processes that can adjust to quickly switching demands while minimising reliance on human involvement. Automated and robotic processes may be necessary to address problems that prohibit people from working together closely9. The fourth industrial revolution is the current era, and it is characterized by the integration of cyber-physical systems (CPS), the internet of things (IoT), and artificial intelligence (AI) into manufacturing. This has enabled the development of smart factories that are highly automated, flexible, and adaptable. These factories can quickly respond to changing market demands and can produce customized products with minimal human intervention. Additionally, Industry 4.0 allows for real-time monitoring and analysis of production data, enabling businesses to make data-driven decisions that can improve productivity and efficiency7-9.
 |
Figure 1: Different stages of the Revolution in the Pharmaceutical IndustryClick here to view Figure |
Key Elements of Industry 4.0 for Pharmaceutical Industries
Internet of Things
The Internet of Things (IoT) is another key technology that enables the connectivity of devices and equipment throughout the manufacturing process. In pharmaceutical manufacturing, IoT devices can be used to monitor critical parameters such as temperature and humidity, ensuring product quality and safety9. IoT devices can also be used to track products throughout the supply chain, providing real-time visibility and enabling timely interventions in case of quality issues. IoT is a revolutionary leader in realm of information technology. The word “Internet” refers to a worldwide network of interconnected computer systems that connects millions of people using the Transmission Control Protocol/Internet Protocol standard suite. A vast array of wireless, digital, and optical networking technologies is used to connect billions of private, public, educational, commercial and government networks globally. RFID is one of the IoT “Things” (Radio Frequency Identification), and other items that, thanks to an unusual addressing system, are able to interact with one another and adapt to their surroundings in order to achieve a common aim10. The first individual who use “Internet of Things” was expert in digital technology, Kevin Ashton. The best way to define the Internet of Things is as “an open wide and informative network of smart devices that can provide information, data, and resources, self-organize, and react and respond in reaction to situations and changes in the environment”. The pharmaceutical industry has grown highly pragmatic in embracing technological advancements, though, so the influence of IoT haven’t felt surely throughout the pharmacy and medical device industries. However, IoT has a significant capacity to assist pharmaceutical companies in improving the production output, reducing prices, and also improving the method of dispensing medications to patients. By monitoring the patient’s compliance and adherence with the recommended prescription in the cloud, IoT closes-loop the physician, manufacturer, distributor and the consumer. With the use of sensors and smart gadgets, the pharmaceutical sector will be able to save more lives and find therapies and efficient care11. On the one hand, IoT boosts the sector’s productivity, resilience, and efficiency; on the other hand, it presents enormous breakthrough opportunities that may help usher in a new era of change in the pharmaceutical industry8-11.
As the first Internet connecting appliance, the Internet of Things (IoT) journey was launched in 1982 at Carnegie Mellon University12. At that time, the Internet Protocol (IP)/Transmission Control Protocol (TCP) was suggested for Internet connectivity in the telecommunications industry. The network’s architecture was established as Web 0.0 in 1990. In 1992, using Online 1.0, the first step toward e-commerce was established for web pages. The term “Internet of Things” (IoT) was first proposed in 2000 for Web 2.0 devices. IoT was introduced in 2010 and was based on technical standardization, to enable complete internet connectivity. Internet developing stages12. Figure 2 represents the development of IoT from its early stages, before 1988, the development of telecommunication devices to present-days prototypes and connected devices.
 |
Figure 2: Stages of IoT developmentClick here to view Figure |
Artificial Intelligence
One of the key technologies of Pharma 4.0 is Artificial Intelligence AI, which has the potential to transform various areas of drug discovery, development, and manufacturing. AI-powered drug discovery tools can accelerate the identification and validation of drug targets, enabling researchers to identify potential new treatments faster and more accurately than traditional methods. AI can also improve the drug development process by predicting the safety and efficacy of new drug candidates, reducing the need for expensive and time-consuming clinical trials12. In manufacturing, AI can optimize production processes by predicting and preventing equipment failures, minimizing downtime, and reducing waste. Additionally, in manufacturing, AI-driven automation streamlines production processes, ensuring higher efficiency and quality control. The use of AI in personalized medicine allows for tailored treatment plans based on individual patient data, optimizing therapeutic outcomes. Overall, AI in the pharmaceutical industry accelerates innovation, increases efficiency, and holds the promise of developing safer and more effective treatments13,14.
Machine Learning
Machine learning (ML) is another technology that plays a critical role in Pharma 4.0. It involves the use of algorithms that can learn from data and make predictions or decisions without being explicitly programmed. In drug discovery, it can be used to analyse large datasets and identify patterns that can help identify new drug targets14-15. In manufacturing, it can be used to optimize production processes by predicting equipment failures and adjusting production schedules accordingly. It is a subset of artificial intelligence (AI) that focuses on the development of algorithms and statistical models that enable computers to perform tasks without explicit programming. In the pharmaceutical industry, it has become increasingly significant due to its potential to revolutionize various aspects of drug discovery, development, and manufacturing. Through the analysis of vast datasets, machine learning algorithms can identify patterns, predict potential drug candidates, and optimize the formulation of new medications. This technology accelerates the drug discovery timeline by enabling researchers to sift through immense biological and chemical data more efficiently, ultimately leading to the identification of promising compounds. Additionally, machine learning facilitates personalized medicine by analyzing patient data to predict individual responses to treatments, optimizing therapeutic outcomes. The integration of machine learning in the pharmaceutical sector not only enhances productivity but also holds the promise of addressing complex medical challenges and fostering the development of innovative, targeted therapies15.
Cloud Computing
Another technology that can enable collaboration and data sharing across the pharmaceutical industry is cloud computing. Cloud-based platforms can facilitate the sharing of data and insights across organizations, enabling more efficient drug discovery and development. Cloud-based systems can also improve supply chain visibility and collaboration, enabling faster response times to quality issues. It has become integral to the pharmaceutical industry, offering a transformative approach to data management, collaboration, and research15 by leveraging cloud services, pharmaceutical companies can streamline their operations, enhance data security, and accelerate drug discovery and development processes. The cloud enables efficient storage and analysis of vast datasets, facilitates real-time collaboration among geographically dispersed teams, and supports the deployment of advanced analytics and machine learning models for predictive modelling and personalized medicine. Additionally, cloud-based solutions enhance scalability, allowing organizations to adapt to changing workloads and demands. This agile and cost-effective approach not only optimizes research and development efforts but also ensures compliance with regulatory requirements, as cloud providers often implement robust security measures. Overall, cloud computing has emerged as a catalyst for innovation within the pharmaceutical sector, fostering agility, collaboration, and accelerated advancements in healthcare16,17.
Big Data Analytics
Big data refers to the vast and complex volume of information that exceeds the capacity of traditional data processing methods. Characterized by the three Vs—volume, velocity, and variety—big data encompasses large amounts of structured and unstructured data, including text, images, videos, and more. The volume aspect signifies the sheer size of the data generated and collected, often in terabytes or petabytes18. Velocity refers to the speed at which this data is generated, processed, and analysed in real-time. Big data analytics involves the use of advanced technologies and algorithms to extract valuable insights, patterns, and trends from these massive datasets, enabling organizations to make informed decisions and gain a competitive edge in various domains such as business, healthcare, finance, and research. In the pharmaceutical industry, big data refers to the vast and complex sets of information generated throughout the drug development and healthcare processes. This includes data from clinical trials, patient records, genomic information, drug interactions, and more. The utilization of big data in the pharmaceutical sector enables researchers and healthcare professionals to gain valuable insights into disease patterns, treatment outcomes, and the efficacy of drugs. By analyzing large datasets, researchers can identify potential drug candidates more efficiently, personalize treatment plans based on patient characteristics, and enhance the overall drug discovery and development pipeline19. Additionally, big data analytics in the pharmaceutical industry contributes to the advancement of precision medicine, allowing for targeted therapies that consider individual variations in genetics, lifestyle, and environmental factors. As the industry continues to embrace big data technologies, it holds the promise of accelerating innovation, improving patient outcomes, and optimizing the overall efficiency of healthcare delivery20.
Pharm 4.0 Ecosystem
The Pharma 4.0 ecosystem includes the new technologies, digital capabilities, the extraordinary expansion of IoT (the Internet of Things), and the impressive ability to reach into data anytime and everywhere in the Supply Chain. Industry 4.0 is evolving the way of product marketing and improving business decisions. Manufacturers are adding new technologies to their production facilities and operations as a whole, like the Internet of Things (IoT), cloud computing and analytics, artificial intelligence (AI), and machine learning. All these technologies, including autonomous real-time collaborative precision robotics and real-time augmented reality, combine together to yield better output for Pharma 4.0 as shown in Figure 3. The combination of artificial intelligence (AI) and robotics makes it possible for systems to run with little to no help from humans21. By combining multiple data sources, it is possible to link information from inside and outside the company. For example, it is possible to combine internal data from the production of pharmaceuticals, like energy and resource management, the results of modelling and simulation, and laboratory data, with external data from other sources, like patient experience, market demand, supplier inventories, and public health emergencies22. To implement Industry 4.0, need to digitise and build security into a number of complex parts of the pharmaceutical supply chain. The industrial internet of things (IoT) is an important idea for building the so-called “smart factory.” It is a type of cyber-physical system made up of computers, sensors, instruments, and other equipment that are all connected and integrated online into a single network. For the Internet of Things to work, data needs to be digitised, which means that data that used to be collected by hand needs to be changed so that it can be collected by digital devices. Information about the manufacturing floor, such as operation procedures and operator work instructions, video monitoring of real-time operations), and video monitoring of real-time operations22.
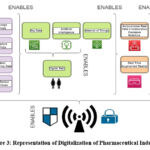 |
Figure 3: Representation of Digitalization of Pharmaceutical IndustryClick here to view Figure |
Advanced Technologies of IoT in Pharm 4.0
IoT is a set of connection of real-world items which depends on networking, sensory, data processing and communicable technologies. It can be thought of as a huge, complicated environment26. The main IoT technology is RFID, which works by allowing microchips to wirelessly send data to the receiver. The implanted RFID-tagged items can be tracked, observed, and analysed using RFID. Pharmaceutical items have been produced, distributed, and sold using RFID technology since the 1980s, and WSN is being used for industrial and healthcare surveillance. The evolution of both technologies is fueling the growth of IoT. Other tools and technologies, like WiMAX, ZigBee and etc., help in data communication, tracking and controlling products, security, and asset of manufacturing process control in the pharmaceutical and health community are mentioned in Table 1.
Table 1: Advanced Technologies in pharmaceutical and health community.
| S. NO. | TECHNOLOGY | USES |
| 1. | (RFID) Radio-frequency identification | Data communication, tracking, and identification. |
| 2. | (WSN) Wireless sensor networks | Track and control the movement, position, noise, and temperature of many different devices.Air pollution, moisture proximity and displacement, speed, pressure, temperature, bird density, infrared, and other variables. |
| 2. | Middleware | To build interactions with RFID tags, sensors, and actuators are all different kinds of devices. |
| 3. | Cloud computing | To create a significant amount of data.To connect IoT and the apps that run on them.Storing, calculating, and transporting for some latency-sensitive. |
| 4. | WiMAX | Provide wireless voice over IP (VoIP).Virtual private LAN Service (VPLS).Provide Surveillance and security system.Sensor networking.Remote monitoring patients. |
| 5. | ZigBee | Asset manufacturing process control.Provide security, HVAC, AMR, light control, access control.Patients monitoring, fitness monitoring. |
Application of IoT in the Pharmaceutical Industry
In Pharmaceutical Value Chain
Even though the pharmaceutical industry is just commencing to use IoT, smart devices and machine-to-machine modern communications are often used to digitise processes and data. IoT is changing the highly regulated pharmaceutical industry by getting rid of old ways of doing things like making medicines and getting them to people. Pharmaceutical firms are now far more willing to experiment with IoT technology in order to increase stakeholder expectations for medicine efficacy while also enhancing quality, increasing productivity, reducing production errors, and lowering costs24. Leveraging IoT in pharmaceutical supply chains, clinical development, and patient involvement not only shortens the time it takes for drugs to reach the market, but it also helps improve quality by finding flaws all along the value chain. Among the popular Internet of Things (IoT) applications, sample lifecycle management, smart packaging, connected materials, people, and connected equipment are several that are especially well suited for many pharmaceutical organisations. The vast majority of pharmaceutical production procedures are normally carried out batches process, and machinery is mainly self-contained. Although industrial automation and control technologies are well-established, executives are unable to use this information to make well-informed decisions to increase overall equipment effectiveness in areas like batch scheduling and maintenance. IoT technology enable businesses to link and increase their visibility into shop activities in order to dramatically increase output and adhere to GMP compliance25.
In Pharmaceutical Manufacturing
Automatic processing software’s and system can be used to control equipment and supplies in addition to improving efficiency in drug manufacture and other relevant tasks, the medicines industry has been adopting batch manufacturing in the past few years. Industrial engineers and other devices may readily get operational data from IoT-enabled equipment, which helps to manage and promotes efficiency. Pharmaceutical industry’s processes include the extraction, manufacture, quality control, and packaging of finished drug products26.
Manufacturing Process
The manufacturing process consists of two key steps: The first is the manufacture of the API (Drug substance) and the second, transformation of the API into the finished pharmaceutical product (Drug Product). A number of unit process and operations, including milling, mixing, granulating, drying, compressing, coating, and packaging, are used to make tablets. With the aid of IoT-based applications, the pharmaceutical industry is able manufacture clinically smart tablets, which is remarkable area of investment. The IoT control system for pharmaceutical production analyses every step of the production process, from the acquisition of raw materials to the packaging of the finished product. The close observation helps to change the system, get rid of lag, and remove effort. Sensors are necessary for every device management system in order to deliver accurate process data. Granulation, milling, coating, and packing are just a few of the processes that are validated as they are being continuously watched over. All environmental aspects of a manufacturing process can be monitored in order to sustain a product quality, which is only possible through production monitoring27.
Manufacturing Environment
IoT greatly help in tracking and monitoring the actual production process from a distance through smart devices and sensors. The surrounding has a significant impact on how pharmaceutical drugs are made. IoT can consequently be used to keep an eye on the weather. Real-time sensors are used by IoT to try to make the medication production process transparent. These sensors collect information on environmental factors like moisture, light, temperature, and radiation can be managed by intelligent devices. To control dropping due to these environmental circumstances, an alert could be set off. IoT sensors allow for the assurance of product quality. Understanding the progression of different stages of the development cycle of product is aided by the data collected by the sensors. The particulars include the basic materials utilised, temperature variations, disposals. The only aspect of a product’s quality that can be changed is how it is being monitored in real-time. Pharmaceutical Internet of Things-based solutions that monitor the manufacturing process aid in upholding product quality28.
Machines and Analytical Instruments
It helps in linking the “Things” that are responsible for output of product, like machines and equipment, in order to increase the efficiency of the production processes. The analog-to-digital converter (ADC) and sensors often provide this function, albeit to a limited extent. As an alternative, we may leverage data that is already being gathered or that is being generated by the goods to make them “smart” and realistically uses the data for growing the business. Pharmaceutical systems, networks, and equipment could be linked and regulated using IoT-based technologies to enhance process standardisation, data gathering, and oversight control. Using sensor-based data, engineers can build predictive models that can learn on their own and add diagnostic capabilities to all of the company’s equipment, making it more efficient, lasting longer, or involving the supply chain less. The manufacturing team is also capable of making sensible decisions to increase resource allocation, decrease downtime, and maximise equipment efficiency, which lowers production costs and reduces cycle times. This is made feasible by gathering real-time data on the calibration, usage, and condition of the equipment. Some of the perceived outcomes of IoT in pharmaceutical industry manufacturing process are lower production costs, monitoring and control in real time, better patient outcomes, and improvement of pharmaceutical unit operations. People use terms like “manufacturing execution system,” “production data,” and “distributed control system” to talk about IoT-based systems29.
In Pharmaceutical Warehousing
The pharmaceutical industry depends heavily on warehousing. Pharmaceutical businesses maintain storage/warehousing facilities across the nation to guarantee a steady and timely supply of their products. Most pharmaceutical firms want to oversee their warehouse function and storage procedures internally, given type products, therefore having their in-house warehouse procedures may be a purposeful move on their part. A McKinsey report claims that 95% of the costs associated with pharmaceutical transportation are attributable to warehousing, making it an expensive business30. Without any real-time view into the activities, tracking goods in warehouse and fully utilising the operators and transport equipment is a difficult process. The usage of IoT in warehouses improves the efficiency and precision of operations including inventory control, sorting and incoming and outside bound shipping. This streamlines the procedure and raises the standard of warehouses services. WSN technology, RFID technology, and wireless video monitoring systems can all be used to manage and oversee warehousing operations overall. When utilised for both inbound and outbound operations, RFID technology effectively boosts the rate of product identification while also performing monitoring and product control. RFID technology performed pallet positioning during inbound and outgoing operations to decrease travel distance, labour costs, and picking distance. Additionally, this made it possible for the receiving system automatically track the location of goods and other data. An automated guided vehicle technique based on RFID was proposed by Lie4,30. It addressed the problem that an AGV could not correctly convey goods to the designated place. The rate of product handling errors both throughout the inbound and outgoing processes was largely reduced as a result. To locate objects, integrated an automobile vision system with RFID application technology. Both inbound and outbound activity productivity have increased dramatically30.
Inventory Control
Monitoring the Inventory at All Stages by the point of arrival to the point of delivery to the consumer, IoT helps to connect the pharmaceutical products. The Internet of Things (IoT) offers information about product expiration dates so that appropriate action can be taken and loss associated with product damage is reduced. By doing this, product waste and spoilage will be reduced30.
Vision Selection
(With Smart Glasses) A type of augmented reality called “vision picking” enables users to work efficiently and without using their hands. This might boost retail productivity. The operators can also view the order selecting instructions on visual display by using smart glasses. The operators or employees of the warehouse just need to have a small amount of training because it is a wearable device; no structural alterations to the warehouse are necessary31.
Analytical Data
Analytical data plan can examine all logistics, trends, and opportunities as the IoT connects. Additionally, real-time visibility made it possible to respond quickly and easily to market changes. Areas in the warehouse that are underperforming may be easily identified and strategies could be made using the information provided by the IoT. Automation tasks drones are used to execute automated tasks. These drones may perform the auditing overnight and provide the data to the operators so they can reassess in the morning. It will prevent operational outages and cut down on costs like labour, electricity, and time that are associated with having human personnel22,31. Lastly, smart warehouses can improve efficiency and visibility by sending measurement results and real-time data to the warehouse staff and management. Sensors are placed in the store room and on the stock items so that they can read and send important data (such as location of the product and the number of items in stock) and warn of troubles, such as lost products. With access to real-time information and 3D views of warehouse events, warehouse managers can monitor and track the storage of highly sensitive drugs in the right places, find problem areas, and assign resources to problems that need human help29-31.
In Supply Chain Management
Pharmaceutical department is strongly linked to both healthcare and medical research, but it also plays a crucial role in production, supply chain management, and transportation. In contrast to the typical supply chain network, this supply chain management has preferences and critical sharp ends because of the unique environmental conditions in which pharmaceutical items must be handled. The phases of the fundamental pharmaceutical supply chain include manufacturers, retailers, hospitals/pharmacies, and consumers22,31. It is similarly important that product requirements are not changed as products are transferred from manufacturing facilities to customers in the case of pharmaceutical medications and medical supplies. In this case, real-time visualisation of transport and warehousing processes is achieved by network analysis and remote monitoring. IoT is ready to change the way a supply chain operates by enhancing both revenue prospects and operational effectiveness. Tracing the assets, prediction and inventory, operator relationships, connected feet, revenue opportunities and maintaining planning are all part of operational efficiency23,31. The pharmaceutical supply chain has various goals that must be met, including:
Suppliers of raw materials must make sure that all ingredients and raw materials sent to pharmaceutical companies are labelled.
Tags shall be scanned and entered into the data-base during production process.
Raw materials each batch is given a special number that includes details about the date of manufacture, the expiration date, and dosage recommendations.
The RFID software provider analyse the tag data and transforms it into an electronic form.
As described in Figure 4. The general overview of the application of the IoT in the pharmaceutical industry, from generating and collecting data from various sources i.e., market and customer needs and implementation of these data in the different parameters of the manufacturing process.
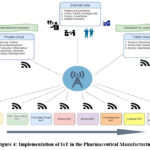 |
Figure 4: Implementation of IoT in the Pharmaceutical ManufacturingClick here to view Figure |
Challenges
Challenges in Regulatory
Regulatory barriers in existing regulatory framework causing challenges for development of Industry 4.0. Lack of acceptance towards technical innovations lead to maintain the conventional processes in industry even though new technologies reduce overall burden of regulatory bodies. Different regulatory bodies over the world have varying regulatory expectations, which lead to another challenge for new technologies to fulfil their needs. The US FDA has been moving forward for performance-based regulations, which is based upon measurable expected outcome rather than dictatorial technologies. This regulation is based on particular set of results, regulatory actions focused on identifying results that ensures safety margin. Industry 4.0 may also ensure the manufacturer and regulatory to identify and focused on the critical point of product to achieve the acceptance limit, which enables industry to improve and evolve as per growing needs and optimizing various critical control parameters with quality product services6,11,31.
The key challenges for regulatory will be controlling and monitoring the risks with continuous change of critical parameters with no or less regulatory surveillance. For Industry 4.0 ecosystem, advanced regulatory and advanced manufacturing technologies need to be brought together for real-time quality assurance for products in performance-based regulations. The on-going process validations approaches includes process design, validation of process qualification and continued process verification over a product lifecycle. Nevertheless, all these process validation steps can be achieved in Industry 4.0 with huge set of data and ability to collect data in continuity, which also provide confidence and accelerate product availability in market. Although challenge will be compiling huge data provided from AI and digitalization. As we know, Pharmaceutical companies are still understanding Industry 3.0 and its advanced technologies PAT and some industries are still stick to in Industry 2.0. Therefore, it will be necessary to set a regulatory framework which is flexible to all the industries for co-existence of old and new technologies to support novel technologies but not impacting product supplied Fifrom older technologies30-31.
Challenges in Technology
Current process validation strategies strive to gather evidence to demonstrate that an operation is in a regulated state and can reliably make great products for the market for the life of a drug (FDA, Guidance for Industry). In order to do this, a sequence of process qualification steps is taken after the process designing phase at the outset of the product ’s life cycle. With persisting process verification, which tries to drive process improvement over the life of the process, these actions give a snapshot of how the process is doing during the launch stage. In an Industrial revolution 4.0 setting, where huge amounts of data are easily accessible and can be analysed, all three cycles of process validation could happen early17,31.
One of the challenges of the technology is that it can be hard to decide how to use the information and insights that big data generates. This information and insights could be used for internal auditing, product release decisions, marketing, or sharing with regulatory organisations. Big Data is the IoT’s platform for machines and devices to interact, talk to each other, and learn from each other. Byrne (2018), Witkowski (2017), and Matthews (2017). Another most important technical challenges for Industry 4.0 will be to figure out and explain the purpose of the data. Even though data by itself can’t solve problems. Status reports, sensor data, and process controls must all be linked to the value chain11,28,31. To monitor and control systems in the future, the business world might only need plug-and-play compatible hardware and software instead of dashboards and user interfaces. The fact that it’s easy to send digital data could theoretically lead to more collaboration and sharing of knowledge between businesses, while making it easier for regulators to collect data (Strategy&, 2018). Even though it’s helpful to share a lot of information, sensitive digital information needs to be kept safe. It might be important to set standards for how data is collected, stored, analysed, and sent16,11,31.
Challenges in logistics
Logistics will be an issue for Industry 4.0, and sometimes the private sector and the government may fight over the same limited resources. On the way to full implementation of Industry 4.0 techniques, producers and regulators will need to make creative cultural changes to deal with the many records, computer technology, and automation threats they face. To endorse a paradigm shift and manufacturing infrastructural facilities premised on digitally enhanced and integrated enterprise systems that rely on processing capacity, communication systems, cybersecurity, and better controls to work at their best, gaps in knowledge and training will need to be filled. To use AI in making pharmaceuticals, it needs more than just the usual biology, chemistry, and process technical expertise. For example, you would need to know about data science, machine learning and systems engineering, IT, and artificial intelligence (AI). At least at first, regulators and businesses may be competing for the same small pool of talent in these fields. When new training requirements for the workforce come out, there will be no doubt that long-term training initiatives will be needed11,30. AI-based mathematical models are a key part of Pharma 4.0. The first step in implementing the concept of AI control into action is to collect past data from cutting-edge production methods like continuous manufacturing. This is because AI-based solutions must use real data to train their model30. For example, to make an AI-based digital twin of a biological or chemical process, the model must be trained with plenty of historical data that shows how the relationship between process control parameters and process performance characteristics has changed over time. In order to make a complete process, appropriate information must be gathered from each process unit. Businesses might make machine learning easier by sharing this historical process data through co – operative, non-competitive, or innovation technics31.
Future Perspective
Industry 4.0 revolutions which combine advancements in digitalisation, automatic systems, robots, and self-organising computational technologies which have ability to change manufacturing process, logistics and supply chains culture of pharmaceutical companies. Future advanced elements in smart factories will improve production flexibility and agility. If Industry 4.0 is to be completely implemented, improvements and innovations must be made to address challenges and risks gathered from huge computational. Industrialists with regulators are working together to develop the modelling and simulation, data management, sensor systems, data analytics, computational and control methodologies necessary to encourage autonomous, artificial, AI, machine learning and computing infrastructures. The U.S. Food and Drug Administration (FDA) has started a project to figure out and put in place the right changes to the regulatory structure to make new technologies possible. Industry 4.0 brings with it some new laws and regulations. One of these is the management of data-rich environments. The ideas of process validation in complex manufacturing systems are changing, and so is the way regulators watch over these systems after they have been approved (FDA, 2019b). For example, ICH Q13 is working on a standard for the continuous manufacture of therapeutic substances and drug products for both small-molecule and biological products. This will make it easier to get regulatory approval for all of these technologies (ICH, 2018). In furthermore to the regulatory, scientific, and logistical problems that will be caused by a new revolution in the way drugs are made, money will also need to be invested. Some people may find it hard to get started because of the initial costs of building and running new or remodelled facilities. Like any other investment, Industry 4.0 might not pay off right away. As a result of better control, lower error rates, faster responses, and fewer drug shortages in the long run, this new way of making things should encourage the application of new technologies.
Conclusion
The integration of the Internet of Things (IoT) in the pharmaceutical industry is transforming traditional processes, driving innovation, and enhancing efficiency across the value chain. From real-time monitoring of drug production and supply chain optimization to patient adherence and personalized medicine, IoT offers unprecedented opportunities to improve outcomes. However, its adoption comes with challenges, including data security concerns, regulatory complexities, high implementation costs, and the need for robust infrastructure. Addressing these challenges requires collaboration among stakeholders, including pharmaceutical companies, technology providers, and regulators, to ensure secure and scalable IoT solutions. With the right strategies in place, IoT has the potential to revolutionize the pharmaceutical industry, fostering improved healthcare delivery and patient care.
Acknowledgement
The author conveyed special thanks to Mr. Jitender Joshi, President, and Prof. (Dr). Dharam Buddhi, Vice Chancellor of Uttaranchal University Dehradun for their support in publishing this review work.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The author(s) do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials
Author Contributions
Yogita Ale: Conceptualization, Visualization and Writing – Original Draft.
Rekha Verma: Funding Acquisition and Resources.
Nidhi Nainwal: Visualization and Supervision.
Sanjeev Kumar Shah: Funding Acquisition and Resources
Vikash Jakhmola: Visualization and Supervision.
Pankaj Pant: Resources.
References
- Arden N.S, Fisher A.C, Tyner K, Lawrence X.Y, Lee S.L, Kopcha M. Industry 4.0 for pharmaceutical manufacturing: Preparing for the smart factories of the future. International Journal of Pharmaceutics. 2021;602 (1):120554.
CrossRef - Madakam S, Ramaswamy R, Tripathi S. Internet of Things (IoT): A literature review. Journal of Computer and Communications. 2015;3(5):164-73.
CrossRef - Sharma A, Kaur J, Singh I. Internet of things (IoT) in pharmaceutical manufacturing, warehousing, and supply chain management. SN Computer Science. 2020 Jul;1(4):232.
CrossRef - Čolaković A, Hadžialić M. Internet of Things (IoT): A review of enabling technologies, challenges, and open research issues. Computer networks. 2018;144:17-39.
CrossRef - Mehta R, Sahni J, Khanna K. Internet of things: Vision, applications and challenges. Procedia computer science. 2018;132:1263-9.
CrossRef - Li S, Xu L, Wang X, Wang J. Integration of hybrid wireless networks in cloud services-oriented enterprise information systems. Enterprise Information Systems. 2012;6(2):165-87.
CrossRef - Wang L, Da Xu L, Bi Z, Xu Y. Data cleaning for RFID and WSN integration. IEEE transactions on industrial informatics. 2013;10(1):408-18.
CrossRef - Ren L, Zhang L, Tao F, Zhang X, Luo Y, Zhang Y. A methodology towards virtualisation-based high performance simulation platform supporting multidisciplinary design of complex products. Enterprise Information Systems. 2012;6(3):267-90.
CrossRef - Tao F, LaiLi Y, Xu L, Zhang L. FC-PACO-RM: a parallel method for service composition optimal-selection in cloud manufacturing system. IEEE Transactions on Industrial Informatics. 2012;9(4):2023-33.
CrossRef - Lee I, Lee K. The Internet of Things (IoT): Applications, investments, and challenges for enterprises. Business horizons. 2015;58(4):431-40.
CrossRef - López TS, Ranasinghe DC, Patkai B, McFarlane D. Taxonomy, technology and applications of smart objects. Information Systems Frontiers. 2011;13:281-300.
CrossRef - Jamali J, Bahrami B, Heidari A, Allahverdizadeh P, Norouzi F. Towards the internet of things. Springer International Publishing; 2020.
- Lee J, Bagheri B, Kao HA. A cyber-physical systems architecture for industry 4.0-based manufacturing systems. Manufacturing letters. 2015;3:18-23.
CrossRef - Thoben KD, Wiesner S, Wuest T. “Industrie 4.0” and smart manufacturing-a review of research issues and application examples. International journal of automation technology. 2017;11(1):4-16.
CrossRef - Singh M, Sachan S, Singh A, Singh KK. Internet of Things in pharma industry: possibilities and challenges. Emergence of pharmaceutical industry growth with industrial IoT approach. 2020; 195-216.
CrossRef - Li W, Kara S. Methodology for monitoring manufacturing environment by using wireless sensor networks (WSN) and the internet of things (IoT). Procedia CIRP. 2017;61:323-8
CrossRef - Zhou, HJ. Zhang, HL Zhou, Localization of pallets in warehouses using passive RFID system. Journal of Central South University.2015:Vol. 22(8): 3017-3025.
CrossRef - Xue Y, Liu H. RFID-Based Intelligent Storage and Retrieval Systems in Automated Warehouse. Softw.. 2011;6(9):1844-50.
CrossRef - Tejesh BS, Neeraja SJ. Warehouse inventory management system using IoT and open source framework. Alexandria Engineering Journal. 2018;57(4):3817-23.
CrossRef - Ding Y, Feng D. Intelligent warehousing based on the Internet of Things technology. 2nd international conference on advances in artificial intelligence 2018; 51-55.
CrossRef - Chamekh M, El Asmi S, Hamdi M, Kim TH. Context aware middleware for rfid based pharmaceutical supply chain. In 2017 13th international wireless communications and mobile computing conference (IWCMC). 2017; 1915-1920.
CrossRef - Angeles R. RFID technologies: Supply-chain applications and implementation issues. Information systems management. 2005;22(1).
CrossRef - Tajima M. Strategic value of RFID in supply chain management. Journal of purchasing and supply management. 2007;13(4):261-73.
CrossRef - Ruiz-Garcia L, Lunadei L. Monitoring cold chain logistics by means of RFID. Sustainable radio frequency identification solutions. 2010;2:37-50.
CrossRef - Guinard D, Trifa V, Wilde E. A resource-oriented architecture for the web of things. IEEE In 2010 Internet of Things (IOT) 2010; 1-8.
CrossRef - Macaulay J, Buckalew L, Chung G. Internet of things in logistics: A collaborative report by DHL and Cisco on implications and use cases for the logistics industry. DHL Trend Research and Cisco Consulting Services. 2015;439-49.
- Toma I, Simperl E, Hench G. A joint roadmap for semantic technologies and the internet of things. In Proceedings of the Third STI Roadmapping Workshop, Crete, Greece 2009; Vol. 1:140-53.
- Javaid M, Haleem A, Singh RP, Suman R. Artificial intelligence applications for industry 4.0: A literature-based study. Journal of Industrial Integration and Management. 2022;7(01):83-111.
CrossRef - Wang W, Ye Z, Gao H, Ouyang D. Computational pharmaceutics-A new paradigm of drug delivery. Journal of Controlled Release. 2021;338:119-36.
CrossRef - Srinivasan R, Marwaha A, Bansal AK, Pharma 4.0 and its impact on the pharmaceutical industry. Current Drug Metabolism. 2020; Vol. 21(1): 28-36
- Weaver E, O’Hagan C, Lamprou DA. The sustainability of emerging technologies for use in pharmaceutical manufacturing. Expert Opinion on Drug Delivery. 2022 ;19(7):861-72.
CrossRef - Zhang C, Chen G, Tang G, Xu X, Feng Z, Lu Y, Chan YT, Wu J, Chen Y, Xu L, Ren Q. Multi-component Chinese medicine formulas for drug discovery: state of the art and future perspectives. Acta Materia Medica. 2023; 2(1):106-25.
CrossRef








