Arun Prabhu Subramanian1 , Rathakrishnan Samiyappan2*
, Rathakrishnan Samiyappan2* , Balakrishnan Anitha3
, Balakrishnan Anitha3 , Gandhimathi Kaliyamoorthi Ayyadurai4
, Gandhimathi Kaliyamoorthi Ayyadurai4 , Jayaprakash Rajendran2
, Jayaprakash Rajendran2
1Department of Chemistry, National College, Tiruchirapalli, Tamil nadu, India.
2Department of Chemistry, School of Arts and Science, Vinayaka Missions Chennai campus, Vinayaka Mission’s Research Foundation (Deemed University), Paiyanoor, Chennai, Tamil nadu India.
3Department of Physics, Jerusalem College of Engineering, Pallikaranai, Chennai, Tamil nadu India.
4Department of Chemistry, Sri Sairam Engineering College, Sai Leo Nagar, West Tambaram, Chennai, Tamil nadu India.
Corresponding Author E-mail:rathakrishnanmsc@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/3046
Abstract
The ability of thiazole derivatives to alter the activity of numerous metabolic enzymes suggests that they have promising therapeutic applications. Their antimicrobial, antifungal, anti-inflammatory, antioxidant, and antiproliferative properties were also established. The Schiff base, which was formed by combining 2, 4-dihydrxy benzaldehyde with phenyl thiazole amine, was studied using UV, FTIR, 1H, and 13C-NMR. The significant absorption (283 nm) and vibrational peaks at 1625 cm-1 were observed for the imine group. The compound was confirmed by the presence of a free proton and carbon peak following the aromatic peaks. The derivative underwent theoretical and biological evaluations, such as antibacterial, inhibition of alpha amylase, and DPPH scavenging assays. Using an online server, QSAR parameters were predicted for the synthesised molecule and compared with drug likeness using Lipinski five rules. The experimental results are compared with theoretical DFT and docking outcomes. The DFT results revealed the compound's reactivity and decreased hardness feature. Docking interaction score ranges from -5.2 to -11.2 kcal/mol. The antimicrobial activity against the pathogens Acinetobacter baumannii, Methicillin-resistant Staphylococcus aureus, and Staphylococcus aureus was observed between 12 and 15 mm inhibition zone with the minimum inhibition concentration maximum of 150±0.28 µg/mL. Likely, antidiabetic and antioxidant outcomes showed the effective concentration from 428.73±0.32 to 590.36± 0.34 µg/mL. There was excellent agreement with theoretical QSAR and docking values in the prepared Schiff base.
Keywords
Biological studies; DFT; Docking; Di-hydroxy benzaldehyde; Schiff base; Thiazole ring
Download this article as:| Copy the following to cite this article: Subramanian A. P, Samiyappan R, Anitha B, Ayyadurai G. K, Rajendran J. Theoretical and Pharmacological Investigations of Phenylthiazol-2, 4 Dihydroxybenzaldehyde Condensed Schiff Base. Biomed Pharmacol J 2024;17(4). |
| Copy the following to cite this URL: Subramanian A. P, Samiyappan R, Anitha B, Ayyadurai G. K, Rajendran J. Theoretical and Pharmacological Investigations of Phenylthiazol-2, 4 Dihydroxybenzaldehyde Condensed Schiff Base. Biomed Pharmacol J 2024;17(4). Available from: https://bit.ly/3NHzJVi |
Introduction
The field of medicinal chemistry relies heavily on the design and synthesis of organic molecules for therapeutic use. The therapeutic efficacy is enhanced by modifying organic compounds with active functional groups, including metals 1. There are increasing opportunities to research Schiff base compounds and their metal complexes thanks to the expanding field of medicinal inorganic chemistry. There have been many advances in the biological utility of Schiff base (SB) compounds since their discovery in 1864 2. They are crucial for creating new materials in the fields of electricity, mechanics, and biology3. They have encouraged interest in generating new physiologically relevant chemicals. Likely, heterocyclic compounds make up around 60% of all known substances 4. Due to a lack of functionalized heterocyclic building blocks, synthesizing structurally varied pharmacological libraries is difficult. Schiff bases are the end product of condensation between active carbonyl groups and primary amines with azomethine groups (-C=N-), which have been shown to have a wide range of beneficial effects, including those against bacteria, fungi, cancer, free radicals, malaria, inflammation, viruses, and cell proliferation5. Because of imine’s ability to form complex compounds with metal ions at the active site of several metabolic enzymes, Schiff bases are pharmacophores6. The hypothesis also suggests that the nitrogen atom (-C=N-) in imines forms hydrogen bonds with the active centers of cell component proteins, thereby impeding cellular functions. To combat germs, diabetes, cancer, inflammation, and viruses, the thiazole moiety has become an indispensable pharmacophore in the field of drug discovery and development. Schiff base compounds based on thiazoles are believed to bind to the reversible oxygen redox system that results from biological reactions, inactivate many cellular enzymes, and donate hydrogen to free radicals; however, there is no proof that these compounds are antioxidants or antibacterial. Crucially, they may be able to explain their antibacterial activity by blocking processes involved in the generation of aminoacyl-tRNA. The field of medicinal chemistry relies heavily on natural compounds (such Epothilones) that contain thiazole derivatives7. A few examples of these molecules are TMC435350, a clinical candidate for antiviral medicine, and MB06322, a medicine candidate for diabetes. Furthermore, thiazole compounds have demonstrated efficacy in the management of bacterial infections, allergies, and other similar conditions8,9. Fanetaizole, a 2-aminothazole derivative, is anti-inflammatory and a pioneering broad-spectrum antibiotic. A tetrahydrothiazole is a structural component of both penicillin and fanetaizole. Molecular structures containing nitrogen, oxygen, and sulfur as donor atoms exhibited a diverse array of biological activities. Drug design also heavily relies on theoretical computational techniques and software 10-12.
Advances in genomics have allowed researchers to pick a large number of target proteins based on their therapeutic potential, thanks to thorough structure-determination. The significant biological activity or the prediction of structure alterations that lead to improved potency is caused by the discovery of a molecular nature and structural properties. Docking approaches factor in the prediction or computation of ligand structure and orientation with respect to the target’s active site. Nowadays, QSAR and docking software are reducing the time and chemical waste in research. Using the reports, our study synthesized the Schiff base from phenyl thiazole amine and two hydroxyl group substituted benzaldehyde. UV, FTIR, and NMR spectroscopies were performed to learn more to confirm the structure. The prepared substance was carried for biological testing and theoretical study.
Experimental
Materials and Methods
All chemicals were received from sigma Aldrich, India and used as such for synthesis. UV spectrum recorded using UV-Vis 2600, Shimadzu in ethanol. FTIR-vibrational spectra recorded for the KBr pellet of the sample in Shimadzu IR affinity IS. Both 1H and 13C NMR spectra were recorded in Bruker 500 MHz using DMSO-d6 solvent. QSAR properties were obtained from molsoft online server for the submitted structure. This research conducted the theoretical DFT calculation using Gaussian-09 software. Additionally, MOE docking software was used to investigate the molecule’s putative binding capability against 1XCW (Human Pancreatic alpha-Amylase) and 3K0K (Breast cancer type 1 susceptibility protein). Sigma-Aldrich, India supplied the chemicals utilized in the biological research, and they were not further purified before use. Bacteria can acquire antibiotic resistance through morphological and biochemical changes; this was demonstrated when the Schiff base was tested for its inhibitory character on the growth of selected bacteria, including A.b; Acinetobacter baumannii, MRSA; Methicillin-resistant Staphylococcus aureus, and SA; Staphylococcus aureus. The agar well diffusion method was used to conduct antimicrobial sensitivity testing on Mueller-Hinton agar (MHA-Hi medium Mumbai). The results were interpreted based on the Clinical and Laboratory Standards Institute standard tables.
Preparation of 4-(((4-phenylthiazol-2-yl) imino) methyl) benzene-1, 3-diol (PTADOHSB)
The 2-amino-4-phenylthiazole crystals were produced and brought to the Schiff base preparation in accordance with the methods described in the literature 13. In a 250 mL round-bottom flask, a solution containing acetophenone (0.1 mol), and 0.2 mol of thiourea, was treated with 0.25 mol of iodine for the target molecule preparation. The solution was heated to 50oC for a 30 min in a water bath. The reaction was then left to proceed under reflux conditions for 7 h after adding 30 mL of methanol to prevent solidification. Subsequently, 50 mL of water was gradually added while stirring until the solution became clear. In a gentle heating environment, the solution was treated with 0.2 g of charcoal and then filtered through cellite powder. Then, the mixture was treated with 100 mL of ethyl acetate when the filtrate had cooled. Followed by the cooling, the solution was neutralized using ammonium hydroxide solution. The upper organic layer was separated and cooled to 15 to 20oC. The developed white needles of thiazole amine were filtered, washed, and dried under vacuum at 500C. Results from the verification of the melting point were consistent with those from earlier measurements 14. The obtained crystals were carried for Schiff base preparation. 0.1 mol of 2, 4 di-hydroxy substituted benzaldehyde and 0.1 mol of 4-phenyl-2 aminothiazole were taken in 100 mL methanol. After 15 min stirred at room temperature, the reaction was continued for 4 hrs at reflux condition. The reaction was tracked using a thin-layer chromatography (TLC) method using a hexane: ethyl acetate ratio of 60:40. After the reaction was completed, the crude solid was filtered and recrystallized from a 9:1 combination of ethanol and water. Then, it was dried under vacuum after being vacuum dried, and shown in Figure.1.
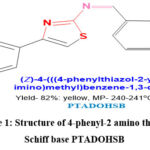 |
Figure 1: Structure of 4-phenyl-2 amino thiazole Schiff base PTADOHSB
|
Characterization of PTADOHSBAbsorption study
The derived PTADOHSB Schiff base was analyzed using absorption spectra. The crystals were dissolved in ethanol and absorption spectra recorded between 200 nm and 450 nm. The obtained spectrum was used to confirm the aromatic character and possible excitations of the attached functional groups.
Vibrational spectra
To confirm the vibrational modes of Schiff base, the vibrational spectrum of the compound was recorded and the functional group change was noticed in a reaction. The main IR bands and their assignments are analyzed for structure.
NMR study
Number of protons and carbons present in derived Schiff base PTADOHSB, both 1H, and 13C NMR spectra were recorded in DMSO –d6 solvents. The recorded spectra are used to confirm the formed imine and aromatic protons. (PTADOHSB): Anal.Calc. for C16 H12 N2 O2 S, 1H-NMR (500 MHz, DMSO-d6) δ 12.03 (s, 1H), 10.28 (s, 1H), 8.85 (s, 1H), 7.99 – 7.94 (m, 2H), 7.49 – 7.40 (m, 4H), 7.19 (s, 1H), 6.55 (d, J = 2.3 Hz, 1H), 6.52 (dd, J = 8.6, 2.3 Hz, 1H). 13C NMR (125 MHz, DMSO-d6) δ 165.28, 164.94, 163.62, 162.60, 151.17, 134.37, 133.24, 129.61, 128.10, 126.93, 114.22, 110.62, 108.63, 104.23, 100.10, 99.58
Quantitative Structure Activity Relationship (QSAR) Studies and Descriptors Profiling
Using the described molecule, we ran a QSAR calculation on the molsoft server, an offline Gaussian-09, and a DFT calculation on Spartan 14. The 2D structure of PTADOHSB was drawn in Chemsketch-2020 and converted to smiles notation. The notation was copied and submitted to molsoft server and predicted the Lipinski rules of five related parameters. Then, the molecular structure has been drawn in both Gaussian-09 and Spartan 14 to calculate the electrostatic potential map, HOMO, and LUMO respectively. Using HOMO and LUMO values, this work calculated the structure related parameters such as chemical potential (μ), hardness (η), softness (S), electronegativity (χ), and global electrophilic index (ω) as per the reports 15,16.
Docking Studies
Using the mcule online server and MOE 2015.10 docking software, the study examined the binding capabilities of the generated compounds against target proteins such as 1XCX (X: 5.2248, Y: 16.4898, Z: 41.577) and 3K0K (X: -23.4849, Y: 56.5041, Z: 2.4167). Initially, the Schiff base structure was drawn in mcule server and docking scores were recorded. The best pose was downloaded and was subjected along with target proteins in MOE software and docked using default settings. After the docking calculations, the binding affinity against the proteins was analyzed, and the 2D interactions of the derived structure were calculated 17. Molecular docking is an intriguing method for researching compound-disease-causing protein interactions to create novel drugs. Binding sites that allow multiple ligand binding may aid medication design.
Antibacterial Activity
The agar well diffusion method was used to evaluate the antibacterial activity 18-20. Using bacterial strains according to the stated procedure, the antimicrobial determination was carried out21. The antibacterial activity of PTADOHSB was evaluated using the agar well diffusion technique in a Mueller-Hinton agar medium. To inoculate the agar plate, a certain amount of microbial inoculum is spread out across the whole surface of the agar. Afterwards, using a sterile cork-borer or a tip, 8 mm diameter hole is aseptically punched. Next, 250 µg/mL of the test sample was placed twice in the same plate along with the Gentamicin standard and DMSO placed as a negative control. After that, the test microbe dictates the parameters that the agar plates are cultured in. By dispersing throughout the agar media, the antimicrobial ingredient impedes the development of the tested microbial strain. Also, this work tested the minimum inhibition concentration by micro-dilution method.
Anti-diabetic Activity
The modified test was used to determine the likelihood that the produced molecule would inhibit α-amylase22-24. 0.5 mg/mL of porcine pancreatic α-amylase was added to 40 μL of sample and 40 μL of 0.02 M sodium phosphate buffer (pH.9 with 0.006 M sodium chloride) before being incubated at 25 °C for 10 min. Every tube was supplemented with 40 μL of a 1% starch solution in a 0.02 M sodium phosphate buffer (pH=9 with 0.006 M NaCl) at 5-second intervals during pre-incubation. The subsequent 10 minutes were spent incubating the reaction mixture at 25°C. An amount of 100 μL of the color reagent made of dinitrosalicylic acid was utilized to stop the reaction. Following that, the tubes were subjected to a water bath heating process until they reached a temperature 5 min above boiling. Afterwards, they were set aside to cool to room temperature. After diluting the reaction mixture with 900 μL of distilled water, the absorbance was measured at 540 nm. Acarbose was used as a reference medicine. In order to determine the percentage of inhibition, the experiments were repeated three times using the following equation eqn (1).
Inhibition (%) = 100 × (control abs –sample abs)/ (control abs)…….. (eqn.1)
Where Abs = absorption. The results were expressed in terms of IC50 representing the concentration of test extracts required to cause the enzyme inhibition by 50%.
Statistical Analysis
All values were expressed mean ± SD. Statistical difference and linear regression analysis were performed using Graphpad prism 5 statistical software.
Antioxidant Assay
The derived thiazole compound capacity to scavenge DPPH free radicals was established by referring to the protocols that have been previously documented 25-27. This work incubated the reaction mixture at room temperature for 60 minutes in the dark with varying doses of 100, 200, 400, 800, and 1600 μg/mL of PTADOHSB and 100 µL of DPPH methanolic solution. At λ = 517 nm, the absorbance of the post-reaction mixture was measured. With gallic acid serving as a positive control, the experiment was repeated triplicated times. The relative absorbance of the control was used to calculate the decrease of DPPH. The following equation (2) was used to compute radical scavenging activity:
% radical assay = [(COD – SOD)/ COD X100] ………….eqn (2)
The IC50 value, which represents the concentration of the test solution needed to inhibit DPPH by 50%, was used to express the results.
Results and Discussion
This section details the biological effectiveness, molecular docking, theoretical QSAR, characterization, and preparation of Schiff bases generated from 2-amino-4-phenylthiazole and di hydroxy benzaldehyde.
Spectral characterization
Structure confirmation was achieved by spectral analysis of the 2-amino-4-phenylthiazole Schiff base. Typical bands observed in the 220-290 nm region were those of the PTADOHSB. Here, the Schiff base displayed two bands, one at 219–230 nm and the other at 268–285 nm28. The hydroxyl and imine groups found in Schiff bases were responsible for these bands. The electron transitions for the π→π* of the aromatic ring caused the produced compound to exhibit an absorption band at 228 nm, while the imine group exhibited a π→π* transition at 283 nm, respectively28. The imine group caused a change in the λmax of the amine, as shown in Figure 2a of the absorption spectra. Further, the compound vibrational spectrum was recorded to confirm the starting material carbonyl functional group disappearance. From the spectrum, the azomethine (-C=N-) group was most likely identified by a prominent band at 1630-1610 cm−1 in the compound’s FT-IR spectra. The thiazole ring’s cyclic (-C=N-) configuration was implicated in the 1567 cm−1 band. In addition to the phenolic C—O section band at 1295 cm−1, there was also a medium band at 775 cm−1 that was identified as the thiazole ring carbon and sulphur bond stretching vibration. A band at 2972 cm−1 was also identified as the presence of an intramolecular hydrogen bond between the phenolic group and the imine-nitrogen atom29. It was shown in Figure 2b that the Schiff base did not have any 2-amino 4-phenyl thiazole amine (-NH2 – 3433, 3250 strong peaks) peaks, according to the infrared spectra plotted from origin30. The wide peaks were also observed in the 3000 – 3500 cm-1 range.
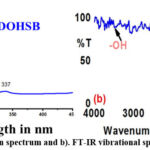 |
Figure 2: a). Absorption spectrum and b). FT-IR vibrational spectrum of PTADOHSB
|
After the basic spectral characterisation, the proton and carbon structural atmosphere was analysed by NMR. At room temperature, the 1H and 13C NMR spectra of the PTADOHSB were performed using DMSO-d6 as the solvent. 1H NMR spectrum of the Schiff base (Figure.3) revealed a single peak at δ 9-12 ppm which was ascribed to the hydroxyl group linked to the benzene ring, while several peaks at δ 7.80-6.93 ppm were caused by aromatic protons.
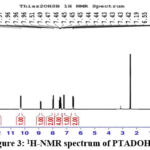 |
Figure 3: 1H-NMR spectrum of PTADOHSB
|
The azomethine proton (-CH=N-) also showed a singlet peak at δ 7.6 – 8.8 ppm. There was a near-perfect agreement between the stated NMR values and the proton NMR chemical shifts. The compound 13C NMR (Figure.4) spectrum showed the signal peak at 159-168 ppm which was attributed to the carbon atoms linked to the hydroxyl group. Because of their similarly-shaped adjacent atoms, a small number of carbons displayed a single peak. The peak was seen between 154 ppm and 159 ppm for the significant azomethine group associated carbon. The structure of the compounds is confirmed by the NMR measurements, which nearly agreed with the published values31,32.
 |
Figure 4: 13C-NMR spectrum of PTADOHSB
|
Theoretical Studies
Table 1 displays the results of calculated Lipinski parameters of the synthesized Schiff base using the molsoft web server (Figure.5a). The molecule was found to meet the drug-likeness (-0.65) criteria and exhibit properties similar to commercially available medications, as shown in the table. A graph showing the drug’s similarity score relative to the standard toxic and drug compounds, along with the percentage of the dataset33. This method can be used to determine the polar surface area of the electronegative atoms that have been substituted. When compared with Kiranmayee (2024) physicochemical property report, our compound has one hydrogen bond acceptor higher than Anastrozole34. Also, our compound showed better drug-likeness when compared with Anastrozole. Simillarly, this work noticed that the reported compound Quercetin exhibit positive drug likeness due to five hydroxyl groups. Hence, hydroxy group has significant pharmacological activity. Further, this research conducted the DFT calculation to support the online outcomes and with theoretical QSAR.
Using Gaussian 9, this research calculated the PTADOHSB HOMO-LUMO energy gap. Several factors pertaining to biological efficiency were determined in terms of the Hatrees unit from the energy gaps of the HOMO and LUMO, following the previously published formulas35. In addition, the method was used to compute the molecular hard and soft natures. The hardness and gap were both reduced in PTADOHSB. With two hydroxyl and one imine functional group, PTADOHSB exhibited a very pliable quality. The important values of electron affinity are represented by the electrophilicity index (ω). There is a clear correlation between the antioxidant properties of compounds and their electron affinity36. Based on the results of the DFT calculations (Figure.5b), this study extracted a subset of attributes pertinent to the biological assessment. Various functional groups in PTADOHSB contribute to its remarkable QSAR capabilities, as seen in the results.
Then, the next step was to determine the Schiff base’s electrostatic potential and use the 3D electrostatic potential function to identify its chemical activities. The red area represents the high potential area, and the blue area represents the low potential area in the MEP image of PTADOHSB, as shown in Figure 5c. The calculated theoretical parameters are presented in Table.1. Hence, the compound was carried for further binding ability with proteins through docking studies against pancreatic alpha-amylase and breast cancer type 1 susceptibility proteins.
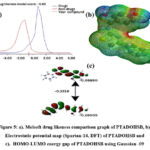 |
Figure 5: a). Molsoft drug likeness comparison graph of PTADOHSB, b). Electrostatic potential map (Spartan-14, DFT) of PTADOHSB and c). HOMO-LUMO energy gap of PTADOHSB using Gaussian -09
|
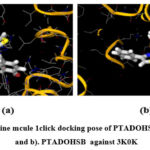 |
Figure 6: a). Online mcule 1click docking pose of PTADOHSB against 1XCX, and b). PTADOHSB against 3K0K
|
Table 1: Online molsoft server and offline DFT QSAR properties of PTADOHSB
|
QSAR properties |
Molsoft |
DFT |
DFT parameters |
Values in eV |
|
Mol. formula |
C16H12N2O2S |
C16H12N2O2S |
HOMO |
-0.28 |
|
Mol.weight (≤500) |
296.06 |
296.35 |
LUMO |
-0.06 |
|
HBA*≤10 |
5 |
5 |
EHOMO+ ELUMO |
-0.34 |
|
HBD*≤5 |
2 |
2 |
E.negativity (χ) |
0.17 |
|
LogP (-0.4 to 5.6) |
4.33 |
0.42 |
Chem.potential (μ) |
-0.17 |
|
LogS (moles/L) |
-4.22 |
— |
ELUMO– EHOMO |
0.22 |
|
PSA (A2) <140 Ǻ2 |
52.31 |
42.124 |
G.Hardness (η) |
0.11 |
|
Vol(A3) |
261.43 |
— |
Softness (S) |
4.52 |
|
MAX EPOT |
— |
314.97 |
Electr. index (ω) |
0.13 |
|
MIN EPOT |
— |
-222.57 |
Softness (σ) |
9.04 |
|
Drug- likeness |
-0.65 |
— |
— |
— |
|
Blood Brain Barrier (BBB) |
3.97 |
— |
— |
— |
*HBA, HBD- no fractions
Docking of the synthesized heterocyclic Schiff base derivative was carried out with PDB ID: 1XCX and PDB ID: 3K0K to predict the preferred orientation of the compounds inside the protein in the mcule docking server (Figure.6 a&b) and the scores are presented in Table.237,38. The negative docking scores and its binding ability are again confirmed by MOE docking software.
Table 2: Mcule online docking scores of PTADOHSB
|
Protein ID |
Ligand |
Mcule Docking score in kcal/mol |
|||
|
1XCX (Diabetic) |
PTADOHSB |
-7.6 |
-7.2 |
-6.9 |
-6.7 |
|
1XCX (Diabetic) |
Clopidogrel (Drug) |
-7.1 |
-6.7 |
-6.7 |
-6.5 |
|
3K0K (cancer) |
PTADOHSB |
-6.1 |
-5.6 |
-5.5 |
-5.2 |
|
3K0K (cancer) |
Dabrafenib (Drug) |
-7.6 |
-7.3 |
-7.1 |
-6.8 |
Docking of the compound was analyzed in terms of hydrogen bonding, energy, non-covalent, and hydrophobic interaction between PTADOHSB and proteins in MOE software39. Docking studies were conducted, as indicated above in the experimental section, and the forces at work in the protein-ligand appreciation, including Vanderwalls bonding via hydrogen in the active site, were assessed. Docking yielded different docking scores for PTADOHSB versus the targets, as shown in Table 3.
Table 3: MOE Docking results of PTADOHSB and standard drugs
|
PDB |
s |
rmsd |
E_conf |
E_place |
E_sc1 |
E_ref |
E_sc2 |
|
1XCX+Comp
|
-5.2 |
3.9 |
-0.1 |
-74.3 |
-11.1 |
-21.4 |
-5.2 |
|
-5.0 |
7.6 |
1.6 |
-79.1 |
-11.2 |
-30.0 |
-5.0 |
|
|
-5.0 |
4.1 |
-4.8 |
-77.0 |
-10.8 |
-19.9 |
-5.0 |
|
|
Clopidogrel |
-5.8 |
11.16 |
-178.3 |
-75.09 |
-9.9 |
-34.5 |
-5.8 |
|
3K0K+Comp |
-4.7 |
7.4 |
3.8 |
-57.4 |
-10.0 |
-1.0 |
-4.7 |
|
-4.5 |
8.2 |
-4.4 |
-52.6 |
-9.6 |
-23.7 |
-4.5 |
|
|
-4.4 |
7.7 |
-2.6 |
-51.8 |
-9.0 |
-23.1 |
-4.4 |
|
|
-4.4 |
7.4 |
-0.1 |
-60.4 |
-9.1 |
-24.5 |
-4.4 |
|
|
Dabrafenib |
-5.5 |
6.634 |
70.37 |
-60.9 |
-10.8 |
-20.5 |
-5.6 |
It was discovered through docking tests that the binding sites that were previously defined had fairly strong affinity with the targets of interest. Docking interaction and images are shown from Figures 7a to 7b.
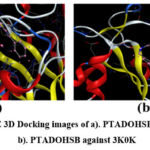 |
Figure 7: MOE 3D Docking images of a). PTADOHSB against 1XCX, b). PTADOHSB against 3K0K
|
The docked PDB files were opened through the MOE docking software and 2D ligand interactions were drawn and presented in Figure.8a and 8b.
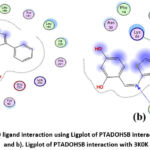 |
Figure 8: a). 2D ligand interaction using Ligplot of PTADOHSB interaction with 1XCX and b). Ligplot of PTADOHSB interaction with 3K0K
|
Biological studies of PTADOHSB
This work proceeded to assess the biological use of the produced thiazole-2, 4-dihydroxy Schiff base. The theoretical results are used to conduct biological experiments to identify the biological efficacy. Initially, the antibacterial activity of thiazole Schiff base was examined against the selected bacterial pathogens, which are shown in Figure.9a, b and c. The antibiotic properties of the Schiff base PTADOHSB, which contains a phenyl thiazole condensed di-hydroxyl group, were determined using the agar well diffusion technique in accordance with the NCCLS criteria. The media’s inhibition zones were measured in millimeters using a zone reader after the specified time period. The anti-microbial test findings showed that the thiazole Schiff bases had a higher level of specificity against the chosen stains, which is in line with what was previously reported. Table 4 shows the findings of the antibacterial susceptibility test. An approach to determining the MIC was by using known technique. Using the following serially diluted concentrations between 3.90 and 500 µg/mL, the test solutions were progressively diluted according to the techniques that were given. Same table shows the results of determining the inhibition concentration using the diluted samples.
Table 4: Antibacterial susceptibility test of PTADOHSB at 250 µg/mL against pathogenic Gram-negative and positive bacteria
|
Compound |
A. baumannii |
MRSA |
S. aureus |
|
PTADOHSB |
12 ± 0.40 (125±0.31) |
12 ± 0.33 (125±0.44) |
15± 0.21 (100±0.27) |
|
DMSO (-ve) |
N |
N |
N |
|
Std. Gentamicin |
15 ± 0.21 (125±0.32) |
16 ± 0.5 (150±0.28) |
15 ± 0.19 (150±0.33) |
The statistical deviation was computed from the triplicated trails and shown in the same table. The synthesized chemical was shown to have particular activity against AB, MRSA, and SA, according to the study. The results were better, and the resistance concentration was the same for PTADOHSB when compared with the gentamicin standard.
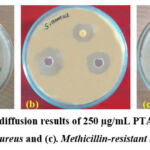 |
Figure 9: Agar well diffusion results of 250 µg/mL PTADOHSB against (a). A.baumannii, (b). S. aureus and (c). Methicillin-resistant Staphylococcus aureus
|
Then, the compound was carried for further efficacy studies. In order to identify the effective concentration, the test sample was diluted in the following order: 100, 200, 300, 400, and 500 µg/mL. It was then sent for the α-amylase inhibition assay40-42. The triplicate trials were carried out, and Table.5 displays the findings of the best trials. Using the outcomes, an online regression graph (https://www.aatbio.com/tools/ic50-calculator) was plotted, and IC50 was calculated (Figure.10). Similar concentrations were used for the DPPH free radical antioxidant assay (Figure.10) and compared with blank43. Trial results are presented in the same table with calculated IC50.
Table 5: Inhibition % of active Schiff base by α-amylase and antioxidant assay
|
α-amylase assay |
DPPH assay |
|||||
|
Std. (µg/mL) |
Inh % |
Con.(µg/mL) |
Inh % |
Con.(µg/mL) |
Abs. |
Inh % |
|
20 |
4.1 |
100 |
46.51 |
100 |
0.48 |
23.81 |
|
40 |
17.8 |
200 |
53.06 |
200 |
0.39 |
38.09 |
|
60 |
32.3 |
300 |
57.4 |
300 |
0.25 |
60.32 |
|
80 |
45.2 |
400 |
61.01 |
400 |
0.14 |
77.78 |
|
100 |
61 |
500 |
64.06 |
500 |
0.03 |
95.24 |
|
Control |
0.23 |
Blank |
— |
Blank |
0.63 |
— |
|
IC50 in µg/mL |
217.67± 0.24 |
590.36± 0.34 |
IC50 |
428.73±0.32 |
||
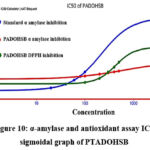 |
Figure 10: α-amylase and antioxidant assay IC50 sigmoidal graph of PTADOHSB
|
Initial QSAR investigation exposed the compound’s drug suitability and found no violation of Lipinski’s five rules. A drug likeness score of -0.65 was shown in the PTADOHSB graphic, which is lower than the value for amoxicillin (1.34). The derivative differs from the amoxicillin molecule in that it has five hydrogen bond acceptors and two hydrogen bond donors. The sulfur, nitrogen, and oxygen atoms that make up our compound all have electronegativity. It follows that the Schiff base was utilized for the offline DFT computation using Spartan-14 in order to determine the polar surface area and the violation of the Lipinski five rules. The Compound exposed the binding energies between −7.6 and −6.7 kcal/mol against pancreatic alpha-amylase protein 1XCX. Likely, it showed heist binding activity with 3K0K which has the binding energies between -6.1 and -5.2 kcal/mol. Leucine, serine, isoleucine, lysine and aspartic acids are interacted with the ligand functional groups. These interactions may have caused the good experimental outcomes. The results were compared with the standard commercial drugs and revealed the nearby coincidence. The antimicrobial outcome suggested that the Schiff base exhibited MIC for a maximum of 150 µg/mL against selected bacterial strains. Based on the results, PTADOHSB effectively inhibited the enzyme at 590.36 ± 0.34 µg/mL. When compared with standard, double the volume of concentration required for the 50% inhibition. This revealed that the compound may be nontoxic in nature. Therefore, a molecule with a high concentration of hydrogen bonds may be an effective antidiabetic, as it exhibited an IC50 at low concentrations. Similarly, antioxidant activity revealed that the compound PTADOHSB exhibited an IC50 value of 428.73 ± 0.32 µg/mL which are almost nearby the concentrations of the α-amylase assay result. Again, the molecule has proved its anti-oxidant activity. From the results, this research work observed the compound’s antidiabetic and antioxidant tendency due to the donor functional groups such as the thiazole ring, hydroxyl group and imine. Over again, thiazole compound proved its biological tendency as like reported in research works. There was a high degree of concordance between the experimental and theoretical values. The effective inhibition is being caused by the PTADOHSB which are the good binding ligands. These results will educate the researchers in drug design in the future. Our compound showed a lower energy gap with more reactivity when compared with reported results44.
Conclusion
This article details the structural confirmation of thiazole Schiff bases by their synthesis and extensive characterization by means of UV, 1H, and 13C NMR. Both online and offline programs were used to compute the theoretical investigations of the chemical structures. Additional experimental assessments of the QSAR properties, chemical reactivity, and molecular softness were carried out by this research, which was a success. The compounds’ protein-binding capabilities with different targets were subsequently assessed through the use of online and offline docking. The efficiency of the test molecule was revealed by the docking results, which were then used for the biological evaluations. Despite experimental evidence supporting PTADOHSB, theoretical results nearly favored compounds based on descriptors. Because of its two hydroxy functional groups connected in an aromatic ring and its inclination to donate hydrogen bonds, the molecule exhibited good biological efficacy. The research shows that the Schiff base has α-amylase inhibitory activity, which could be used to treat Type 2 diabetes by reducing postprandial hyperglycemia after in vivo study in future. Evidence of antioxidant activity suggests that PTADOHSB, with a little structural change, could be a future therapeutic candidate. An acceptable method for estimating the effect of substituents on the antioxidant activities of all produced compounds, the DPPH radical scavenging assay demonstrated the necessary and desirable activity.
Acknowledgement
The authors’ wishes to show his deepest gratitude to Dr. R. Jayaprakash, Associate Professor, SAS, VMRF Chennai Campus, VMRF (DU) for allow us to continue his work and invaluable knowledge support during our entire investigation, as well as for his contributions to the manuscript.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The author(s) do not have any conflict of interest
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials
Authors’ Contribution
Arun Prabhu Subramanian: Conceptualization, biological work
Rathakrishnan Samiyappan: Supervision
Balakrishnan Anitha: Visualization, English correction.
Gandhimathi Kaliyamoorthi Ayyadurai: Analysis.
Jayaprakash Rajendran: Methodology, theoretical studies and Writing
References
- David J, Newman G, Cragg MK, Snader M. The influence of natural products upon drug discovery. Nat Prod Rep. 2000; 18: 215.
- Boutin JA, Lambert PH, Bertin S, Volland JP Fauchere JL. Physico-chemical and biological analysis of true combinatorial, Libraries. J Chrom B. 1999; 725:17-37.
CrossRef - Elmali A, Kabak M Elerman Y. Keto-enoltautomerism, conformations and structure of N-(2-hydroxy-5- methylphenyl), 2-hydroxybenzaldehydeimine. J Mol Struct. 2000; 477: 151-158.
CrossRef - Sortino M, Delgado P, Juárez S, Quiroga J, Abonía R, Insuasty B, Nogueras M, Rodero L, Garibotto FM, Enriz RD, Zacchino SA. Synthesis and antifungal activity of (Z)‑5‑arylidenerhodanines. Bioorg Med Chem Lett. 2007; 15:484-94.
CrossRef - Parvez A, Jyotsna M, Vandana T, Microwave Mediated Cyclo condensation of 2-amino thiazole into β-lactam Derivatives: Virtual Screening and In Vitro Antimicrobial Activity with Various, Microorganisms. Int J chemtech Res. 2010; 2:956-964.
- Kajal Anu, Bala Suman, Kamboj Sunil, Sharma Neha, Saini Vipin. Schiff Bases: A Versatile Pharmacophore. Journal of Catalysts. 2013; 2013:1–14. https://doi.org/10.1155/2013/893512.
CrossRef - Mokhles M, Abd-Elzaher A, Ammar A, Labib A, Hanan A, Mousa A, Samia A, Moustafa A, Mamdouh M, Ali B, Ahmed A El-Rashedy. Synthesis, anticancer activity and molecular docking study of Schiff base complexes containing thiazole moiety, Beni-Suef. Uni J Bas App Sci. 2016;5:85-96.
CrossRef - Brzezin S, Kos K, Walczyn SK. Application of thin-layer chromatographic data in quantitative structure–activity relationship assay of thiazole and benzothiazole derivatives with H1-antihistamine activity. J Chromatogr. 2003;1007:145-155. https://doi.org/10.1016/ s0021-9673(03)00961-0.
CrossRef - Suzuki N, Shiota T, Watanabe F, Haga N, Murashi T, Ohara T, Matsuo K, Oomori N, Yari H, Dohi K, Inoue M, Iguchi M, Sentou J, Wada T. Synthesis and evaluation of novel pyrimidine-based dual EGFR/Her-2 inhibitors. Bio Org Med Chem Lett. 1994;4:1601-6.
CrossRef - Ferreira LG, Dos Santos RN, Oliva G, Andricopulo AD. Molecular docking and structure-based drug design strategies. Molecules. 2015;20(7):13384-421.
CrossRef - Abrar Bayazeed RB, Alnoman Kahdr Alatawi OM, Alatawi AM, Alqahtani Mariam Mojally, NA, Alenazi NM, El-Metwaly. Synthesis, molecular modeling and bioactivity of new bis-thiazole, thiazole-pyrazole, and thiazole-pyridine analogues, Journal of Saudi Chemical Society. 2023;27(6):1-12.
CrossRef - Sliwoski G, Kothiwale S, Meiler J, Lowe EW Jr. Computational methods in drug discovery. Pharmacol Rev. 2013;66(1):334-95.
CrossRef - Pouralimardan AO, Chamayou ACB, Janiak BC Monfared AHH, Hydrazone Schiff base-manganese (II) complexes: Synthesis, crystal structure and catalytic reactivity. Inorg Chimi Acta. 2007; 360: 1599-1608.
CrossRef - Bakht MAA, Yar SBM, Hamid SGA, Saleh I, Al Qasoumi A Samad A. Molecular properties prediction, synthesis and antimicrobial activity of some newer oxadiazole derivatives, Eur J Med Chem. 2010;45:5862-5869.
CrossRef - G. K. Ayyadurai, R. Jayaprakash, “Theoretical studies on covid 19-3jcl spike protein and docking study with Azadirachta Indica nimbin and nimbolide”, International journal for innovative research in multidisciplinary field. 2021;7(4):24-29.
- Ayyadurai G, Jayaprakash R, Rathika S. Theoretical Molecular Properties Prediction, Docking and Antimicrobial Studies on Anisidine-Isatin Schiff Bases. Asian Journal of Chemistry, 2021;33(12): 3025–3030. https://doi.org/10.14233/ajchem.2021.23467.
CrossRef - Ayyadurai GK, Jayaprakash R, Shajahan A, Rathika S. Studies on 2-((2, 4-dihydroxybenzylidene) amino)-3-phenylpropanoic acid include antimicrobial, antidiabetic, antioxidant, anticancer, hemolysis, and theoretical QSAR, Journal of Biomolecular Structure and Dynamics. 2023; https://doi.org/10.1080/07391102.2 023.2294383.
CrossRef - Ayyadurai, G. K., Jayaprakash, R., Shajahan, A., & Rathika, S. (2023). Studies on 2-((2, 4-dihydroxybenzylidene) amino)-3-phenylpropanoic acid include antimicrobial, antidiabetic, antioxidant, anticancer, hemolysis, and theoretical QSAR. Journal of Biomolecular Structure and Dynamics, 1–13. https://doi.org/10.1080/07391102.2023.2294383.
CrossRef - Jayaprakash R, Saroj Kumar S, Hemalatha S, Easwaramoorthy D. QSAR, brine shrimp lethal assay and antimicrobial studies on synthesized L-tryptophan-2, 4-dihydroxy benzaldehyde Schiff base. Inter J ChemTech Research. 2016; 9 (6): 48-54.
- B. Preethi, Sarojkumar Sha, R. Jayaprakash, S. Kutti Rani and S. Hemalatha, “Synthesis, characterization and biological studies on 4-bromo-2-{(z)-[(furan-2-ylmethyl)imino]methyl}phenol praseodymium complex”, Rasayan J. Chem., 12(3), 1455-1462(2019). http://dx.doi.org/10.31788/RJC.2019.1235296.
CrossRef - Semysim E. A. R. A. Evaluation of Antibacterial Activity of Extracts of Curcuma Longa L. Rhizome and Estimation of Curcuminoid by HPLC. Biomed Pharmacol J 2022;15(4).
CrossRef - Janusz S, Anna J, Maciej H, Grzegorz K, Barbara M, Elżbieta M and Jacek S. Characterization and antidiabetic activity of salicylhydrazone Schiff base vanadium(IV) and (V) complexes. Transit Met Chem. 2021;46:201–217.
CrossRef - Abd-Elzaher Mokhles M, Labib Ammar A, Mousa Hanan A, Moustafa Samia A, Ali Mamdouh M, El-Rashedy, Ahmed A. Synthesis, anticancer activity and molecular docking study of Schiff base complexes containing thiazole moiety. Beni-Suef University Journal of Basic and Applied Sciences. 2016;5: 85-96.
CrossRef - Loh SP, Hadira O. In vitro inhibitory potential of selected Malaysian plants against key enzymes involved in hyperglycemia and hypertension. Malays J Nutr. 2011;17: 77–86.
- Uzma S, Khalid MK, Sridevi C, Muhammad T, Abdul W, Shantini V, Mehreen G and Shahnaz P. New Hybrid Hydrazinyl Thiazole Substituted Chromones: As Potential α-Amylase Inhibitors and Radical (DPPH & ABTS) Scavengers. Sci Rep. 2017; 7: 16980.
CrossRef - Karekal MR, Biradar V, Bennikallu Hire Mathada M. Synthesis, Characterization, Antimicrobial, DNA Cleavage, and Antioxidant Studies of Some Metal Complexes Derived from Schiff Base Containing Indole and Quinoline Moieties. Bioinorganic Chemistry and Applications. 2013; 2013:1–16. https://doi.org/10.1155/2013/315972.
CrossRef - Smolyaninov IV, Poddel’sky AI, Baryshnikova SV, Kuzmin VV, Korchagina EO, Arsenyev MV, Smolyaninova S.A.; Berberova, NT. Electrochemical transformations and evaluation of antioxidant activity of some Schiff bases containing ferrocenyl and (thio-)phenol, catechol fragments. Applied Organometallic Chemistry. 2017; 32(3),1-14. https://doi.org/10.1002/aoc.4121.
CrossRef - Sanchez Moreno C, Larrauri JA, Saura-Calixto F. A Procedure to Measure the Antiradical Efficiency of Polyphenols. Journal of the Science of Food and Agriculture, 1998;76: 270-276.
CrossRef - Vinusha HM, Shiva Prasad K, Chandan S, Muneera B. Imino-4-Methoxyphenol Thiazole Derived Schiff Base Ligands: Synthesis, Spectral Characterization and Antimicrobial Activity. Chem Sci J. 2015;6:1-4.
CrossRef - Nazır H, Yıldız M, Yılmaz H, Tahir MN, Ulku D. Intramolecular hydrogen bonding and tautomerism in Schiff bases structure of N-(2-pyridil)-2-oxo-1-naphthylidenemethylamine, J. Mol.Struct. 2000;524: 241-50.
CrossRef - Rageh HM, Ibrahim SA, Selim MA, Alsoghier HM. Spectroscopic and semi empirical investigation of the structural features of hetarylazo-5-isoxazolones tautomerism. J Saudi Chem Soc. 2017;21: S467-S474.
CrossRef - Amra ZH, Yassir S. Al-Jawaheri and Amaal YA, Assafe, Synthesis of substituted heterocyclic with their cobalt (II) complexes from 2-aminothiazoles and evaluation of their biological activity, Bull. Chem. Soc. Ethiop. 2024; 38(4): 909-922.
CrossRef - Soham B, Adrish D, Pijush KK, Neha G, Ritesh D, Nikolay TT, Luigi M, Maria P, In silico exploration of 4(α-l-rhamnosyloxy)-benzyl isothiocyanate: A promising phytochemical-based drug discovery approach for combating multi-drug resistant Staphylococcus aureus, Computers in Biology and Medicine, 179, 2024,108907, pp.1-17. https://doi.org/10.1016/j.compbiomed.2024.108907.
CrossRef - Kiranmayee P. The Binding Capacity of the Lead Phytochemical Molecule to Cancer Cell Target Proteins and its Potential Anticancer Properties with Respect to Standard Drugs. Biomed Pharmacol J 2024;17(2), pp.965-997.
CrossRef - Sami AA, Mahmoud SB, Hammed MA, Adel AAE, Tarek AAM. Synthesis, spectroscopic properties, molecular docking, anti-colon cancer and anti-microbial studies of some novel metal complexes for 2-amino-4-phenylthiazole derivative Spectrochim. Acta Part A. 2015;145:425-439.
CrossRef - Mohammad Jahidul Islam AK, Nuruzzaman Sarker SP, Afroza Z. The Prediction and Theoretical Study for Chemical Reactivity, Thermophysical and Biological Activity of Morpholinium Nitrate and Nitrite Ionic Liquid Crystals: A DFT Study. Adv J Chem A. 2019; 2(4): 316-326.
CrossRef - Pop R, Medeleanu M, Andoni M. Investigations Regarding the Antioxidant Character of Some Flavonols. Chem. Bull. “Politehnica” Univ. (Timisoara) 2013;58, 13-15.
- Periasamy J, Kurdekar V, Jasti S, Nijaguna MB, Boggaram S, Hurakadli MA, Raina D, Kurup LM, Chintha C, Manjunath K, Goyal A, Sadasivam G, Bharatham K, Padigaru M, Potluri V, Venkitaraman AR. Targeting Phosphopeptide Recognition by the Human BRCA1 Tandem BRCT Domain to Interrupt BRCA1-Dependent Signaling. Cell Chem Biol. 2018; 25(6):677-690.e12.
CrossRef - Vadiraj K, Saranya G, Jasti S, Mamatha B. Nijaguna, JP, Sanjana B, Kavitha B, Vijay Potluri AV, Shivange GS, Muralidhara P, Ashok RV. Structural features underlying the activity of benzimidazole derivatives that target phosphopeptide recognition by the tandem BRCT domain of the BRCA1 protein, bioRxiv. 555623; 2019;2019:1-30.
- Almarhoon ZM, Al-Onazi WA, Alothman, AA, Al-Mohaimeed AM, Al-Farraj ES. Synthesis, DNA Binding, and Molecular Docking Studies of Dimethylaminobenzaldehyde-Based Bioactive Schiff Bases. Journal of Chemistry, 2019;2019:1–14. https://doi.org/10.1155/2019/8152721.
CrossRef - Balan K, Ratha P, Prakash G, Viswanathamurthi P, Adisakwattana S, Palvannan T. (2014). Evaluation of invitro α-amylase and α-glucosidase inhibitory potential of N2O2 schiff base Zn complex. Arab J Chem.2017; 10(5): 732-738.
CrossRef - Induar S, Dubey D, Rath S, Meher R. K, Swain S. K, Tripathy S. K. Evaluation of the Antioxidant and Antimicrobial Activity of the Nutritionally Rich Plant, Dioscorea alata L. Biomed Pharmacol J 2024;17(2).
CrossRef - Somashekara B, Thippeswamy B, Vijayakumar GR. Synthesis, antioxidant and α -amylase inhibition activity of naphthalene-containing 2, 4,5-trisubstituted imidazole derivatives. Journal of Chemical Sciences. 2019; 131(7): 1-7.
CrossRef - Anza M, Endale M, Cardona L, Cortes D, Eswaramoorthy R, Zueco J, Rico H, Trelis M, Abarca B. Antimicrobial Activity, in silico Molecular Docking, ADMET and DFT Analysis of Secondary Metabolites from Roots of Three Ethiopian Medicinal Plants. Adv Appl Bioinform Chem. 2021;14:117-132.
CrossRef







