Dewa Ayu Agung Alit Suka Astini1,2* , I Wayan Putu Sutirta Yasa3
, I Wayan Putu Sutirta Yasa3 , I Made Jawi4
, I Made Jawi4 , I Nyoman Wande3
, I Nyoman Wande3 , Putu Indah Budi Apsari5
, Putu Indah Budi Apsari5 and Luh Gde Evayanti2
and Luh Gde Evayanti2
1Doctoral Program, Faculty of Medicine, Universitas Udayana, Denpasar, Indonesia.
2Anatomy Departement, Faculty of Medicine and Health Sciences, Universitas Warmadewa, Denpasar, Indonesia.
3Clinical Pathology Department, Faculty of Medicine, Universitas Udayana, Denpasar, Indonesia.
4Pharmacology Department, Faculty of Medicine, Universitas Udayana, Denpasar, Indonesia.
5Parasitology Departement, Faculty of Medicine and Health Sciences, Universitas Warmadewa, Denpasar, Indonesia.
Corresponding Author E-mail: sukesukaastini@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/3059
Abstract
Noise is an environmental condition that we encounter in everyday life. The level of noise varies in certain environments, such as work environments, traffic on highways, noise in cafeterias, and others. This exposure can affect the coordination system in our body, such as the cerebellum. Soursop leaf extract has been widely used in traditional medicine, the antioxidant content in this extract has many health benefits. Soursop leaf extract (Annona muricata L.) can be useful as an anti-inflammatory, providing protection to nerve cells from free radicals, and other benefits. This study aims to test the effect of soursop leaf extract on neurons in the cerebellum layer. The study was conducted at the experimental animal stage. The experimental animals used were adult Wistar rats which were divided into 2 control groups and 1 treatment group. Each group consisted of 15 Wistar rats. The treatment group was given ethanol extract of soursop leaves before being exposed to noise. The results showed that there were significant differences in the density of molecular layer nerve fibers, between K- and K+ groups (p=0.000) and P (p=0.015), between K+ and P groups (p=0.015). There was a significant difference in Purkinje cell degeneration in the control and treatment groups, between K- and K+ groups (p=0.000) and P (p=0.003), between K+ and P groups (p=0.015). There was no significant difference in granular cell degeneration in the control and treatment groups, between K- and K+ groups (p=0.061) and P (p=0.838), between K+ and P (p=0.094), but the degeneration was lighter in P than K+. The conclusion of this study is that soursop leaf extract provides protection to neurons in the cerebellum from noise exposure.
Keywords
Antioxidant; Granular Cells; Neuroprotective; Purkinje Cells; Soursop Leaf
Download this article as:| Copy the following to cite this article: Astini D. A. A. A. S, Yasa I. W. P. S, Jawi I. M, Wande I. N, Apsari P. I. B, Evayanti L. G. The Effect of Ethanolic Extract of Annona muricata L. Leaves on Cerebellum Neurons in Noise-Exposed Adult Wistar Rats. Biomed Pharmacol J 2024;17(4). |
| Copy the following to cite this URL: Astini D. A. A. A. S, Yasa I. W. P. S, Jawi I. M, Wande I. N, Apsari P. I. B, Evayanti L. G. The Effect of Ethanolic Extract of Annona muricata L. Leaves on Cerebellum Neurons in Noise-Exposed Adult Wistar Rats. Biomed Pharmacol J 2024;17(4). Available from: https://bit.ly/4fbdfrq |
Introduction
Soursop leaf extract is obtained from the soursop plant (Annona muricata L.), this plant has been widely used for traditional medicine and is believed to have health benefits. Several studies have previously been conducted on this extract, showing its high antioxidant content and benefits such as anticancer and anti-inflammatory.
This extract’s compounds such as acetogenin, alkaloids and flavonoids can significantly inhibit the growth of cancer cells 1. Soursop leaf extract also has significant antimicrobial activity against pathogens 2. Its benefits as an antioxidant and anti-inflammatory are associated with its potential to protect neurons from oxidative damage. Antioxidants are compounds that can protect body cells from damage caused by free radicals. Previous research has found that soursop leaves contain various antioxidant compounds, such as alkaloids, flavonoids, tannins and phenolics 3.
The human brain has a vital function in the body. The brain functions to control the internal environment and external responses of the human body. One part of the brain is the cerebellum which is responsible for motor coordination and balance. Both the brain and cerebellum are susceptible to external disturbances such as noise.
Noise is an environmental factor that is often considered trivial, but can have an impact on brain health. Previous research has shown that prolonged exposure to noise can cause various neurological problems. Noise can interfere with cognitive function, increase stress levels, and potentially increase the progression towards neurodegenerative diseases 4. The cerebellum can also be negatively impacted by noise. Disorders of the cerebellum can cause problems with human motor coordination and balance. Previous research shows that exposure to noise can disrupt the process of motor learning and adaptation, which is a function of the cerebellum 5. Protection of the brain may include cerebellar function. Chronic inflammation and neuronal degeneration can have a negative impact on the central nervous system including the cerebellum 6,7.Compounds in this extract such as flavonoids and acetogenin have anti-inflammatory and anti-neurodegenerative activities.
Most previous studies tend to focus only on the effects of Annona muricata L. leaves extracts in the context of general oxidative stress, or on other organs, and few have examined its protection of cerebellar neurons exposed to noise. This creates a significant research gap regarding the use of Annona muricata L. leaves extracts for nervous system protection against adverse environmental exposures, such as noise. To address this gap, this study aimed to evaluate the neuroprotective effects of Annona muricata L. extract on cerebellar neurons of adult Wistar rats exposed to noise. This study is expected to provide a deeper understanding of Annona muricata L. protection against noise-induced neuronal damage, as well as enrich the literature on antioxidant-based therapeutic approaches to reduce the impact of oxidative stress on the brain.
Materials and Methods
Preparation and phytochemical analysis of soursop leaves ethanol extract
Soursop leaf extract was obtained from locations that met organic farming standards, and the extraction process and phytochemical tests were carried out at the Integrated Services Laboratory, Faculty of Agricultural Technology, Universitas Udayana. The selected leaves were then cleaned, dried, and ground into powder using a grinder. The simplicia powder is then subjected to an extraction process using the maceration method and 96% ethanol solvent.
Ethanol extract of soursop leaves were analyzed to identify the main phytochemical compounds, namely alkaloids, flavonoids, tannins, phenols and other related compounds. Phytochemical screening methods used include testing with certain reagents, such as the Wagner test for alkaloids, the Shinoda test for flavonoids, and the ferric chloride test for tannins.
To evaluate the ability of soursop leaf ethanol extract in reducing free radical compounds (antioxidant capacity) IC50 using the Ferric Reducing Antioxidant Power (FRAP). To measure the phytochemical content of phenols, flavonoids, tannins, vitamin C, beta-carotene, tocopherol, and saponins using the Colorimetric Method.
Extraction process of soursop
In this study, quantitative analysis of phytochemical compounds including phenols, flavonoids, tannins, vitamin C, tocopherols, and beta-carotene was carried out, as well as evaluation of the quality of saponins in Annona muricata L. leaf extract. This procedure was carried out using a colorimetric method that is appropriate for each compound.
Sample Preparation
Annona muricata L. leaf simplicia to be analyzed was prepared by extracting it with ethanol solvent. This process is carried out to ensure that the desired phytochemical compounds are well dissolved.
Quantitative Analysis with Colorimetric Method
After the extract is obtained, the quantity of phytochemical compounds is measured using various colorimetric methods, namely the phenol content is measured using the Folin-Ciocalteu method. Folin-Ciocalteu reagent is added to the ethanol extract, then the absorbance is measured to determine the phenol concentration. To measure flavonoids, the aluminum chloride method is used, where the extract is mixed with the reagent and the color change is measured spectrophotometrically. Tannin levels were determined using FeCl₃ reagent, where the extracts were mixed and the absorbance was measured to determine the concentration of tannins based on the formation of a color complex. The DCPIP (dichlorophenolindophenol) method was used to measure vitamin C levels, where the extracts were tested with DCPIP reagent and the change in absorbance was measured. Tocopherol levels were measured using a colorimetric method that is appropriate for assessing the concentration of tocopherol in the extract. The spectrophotometric method was used to measure the concentration of beta-carotene by assessing the absorbance at a certain wavelength. For saponins, quality testing was carried out using the Schales method, namely the ethanol extract solution was mixed with water to check for foam formation. Saponins have the ability to form stable foam. Observation of the amount and stability of the foam will provide an indication of the presence of saponins in the sample.
Data Analysis
After all measurements were carried out, data from the colorimetric method was analyzed to calculate the concentration of each phytochemical compound. The results of the saponin quality test will also be recorded to provide an overview of the presence of saponins in the extract.
The antioxidant potential (IC50) of Annona muricata L. leaf extract was measured using the FRAP method, which is the concentration of extract required to reduce 50% of free radical activity.
Sample Preparation
Preparation of ethanol extract of Annona muricata L. leaves
Preparation of FRAP Reagent
This reagent consists of a mixture of acetic acid solution (pH 3.6), FeCl₃ solution, and TPTZ solution (2,4,6-tris(2-pyridyl)-s-triazine) in certain proportions. This mixture is needed to assess the reduction ability of the antioxidant compounds in the extract.
Determination of Extract Concentration
Next, several different extract concentrations were determined, namely 100, 200, 400, 600, 800, and 1000 μg/mL. These various concentrations will be tested to determine the antioxidant activity shown by the extract.
Reaction Process
For each concentration that has been prepared, a certain amount of extract (for example 0.1 mL) is mixed with 2.9 mL of FRAP reagent solution that has been prepared previously. The mixture is then incubated at room temperature for 30 minutes, allowing the reaction between the compounds in the extract and the FRAP reagent to occur optimally.
Absorbance Measurement
After the incubation process, the absorbance of each mixture is measured using a spectrophotometer at a wavelength of 593 nm. This measurement is carried out to determine how effective the extract is in reducing Fe³⁺ to Fe²⁺, which is indicated by changes in absorbance.
Determination of Antioxidant Activity
Antioxidant activity is calculated based on the observed absorbance changes. The lower the absorbance value measured, the higher the reduction activity shown by the compounds in the extract. Data from this measurement are very important for further analysis.
IC50 Calculation
The absorbance data obtained from each concentration are evaluated to create a concentration versus percent activity curve. The IC50 value was determined using regression analysis, namely the concentration of extract required to reduce free radical activity by 50%.
Animal Study Design and Setting
This research was conducted on adult Wistar rats (Rattus norvegicus) as experimental animals. There were 45 experimental animals, divided into 15 experimental animals in each of 2 control groups (K+ and K-) and 1 treatment group (P). Groups K+ and P were given noise exposure of 95dB for 4 hours for 14 days. Group P was given ethanol extract of soursop leaves at a dose of 100mg/kg body weight before being exposed to noise for 14 days. Meanwhile, the K+ group was given distilled water.
Animal Care and Treatment
The research samples were all male Wistar strain white rats (Rattus norvegicus) aged 2-3 months, weighing 100-150 grams, kept at the Integrated Laboratory of Universitas Udayana. Animals are kept and cared for by giving them food and water and keeping their cages clean. Experimental animals were fed ad libitum, both on a high-fat diet and a standard diet. Drinking is also provided ad libitum, in the form of bottled water. Experimental animals were kept in groups in cages measuring 30x40x40 cm, which were made of wire and had sufficient ventilation. Each cage contains 5 experimental animals, where each cage is given a partition for each experimental animal. Every three days the cage is cleaned of dirt and food waste. Lighting is regulated with a cycle of 12 hours of light and 12 hours of darkness (light cycle from 6 am to 6 pm) with a room temperature of 30 ± 10C. For 1 week the animals are tried to acclimate so they get used to living in this environment. The physical health of the test animal can be seen to ensure the animal can make adjustments. Healthy experimental animals can be seen from open and clean eyes, smooth and shiny fur, active activity and good appetite.
Groups K+ and P were exposed to a noise stressor for 4 hours with a sound pressure level of 95 dB from Real-time analyzer software version 5.2.0 (Yoshimasa Electronic Inc., Japan) connected to a loudspeaker (Sony SRS XB30, Japan) for 14 days on a drum soundproof measuring 100 x 100 cm. Group P was given Annona muricata leaf extract 100mg/kg body weight orally once a day before noise exposure. Several previous studies have shown that a dose of Annona muricata L. extract of 100 mg/kg body weight is effective in providing health benefits, such as increasing HDL, reducing various breast cancer markers, inhibiting the growth of prostate tumors, and reducing blood glucose and regenerating pancreatic beta cells 1,8,9,10. The acute toxicity of ethanol extract of Annona muricata L. leaves is more than 2000 mg/kg. Previous studies with mice showed this effect within 2 hours after administration and subsided after 4 hours. The effects on the kidneys and liver were mild and insignificant, indicating that this extract is practically non-toxic 11,12.
Rat Dissection and Brain Organ Removalal
On day 15, the rats were sacrificed by peritoneal injection of ketamine at a dose of 150 mg/kgbb and the brain organs were taken. Brain organs were fixed in 10% buffered formalin solution in plastic containers that had been labeled according to the group and sample number of the experimental animal. After 8 hours (maximum 24 hours), the brain organ is made into a histology preparation using the paraffin method. Paraffin blocks were cut with a rotary microtome with a thickness of 4-5 μm and attached to glass objects, followed by hematoxylin and eosin staining.
Measurement of Effects on Neurons in the Cerebellum
After Hematoxylin and Eosin (HE) staining, detailed cell morphology observations were carried out using a microscope (magnification 40x). Detailed morphological observations include identification of the layers of the cerebellum, namely the molecular layer, the Purkinje layer and the granular layer. In the molecular layer, observations were made on the density of nerve fibers (dendrites). Observations of Purkinje cells and granular layer were carried out for signs of degeneration in the cells.
Results and Discussion
Phytochemical Analysis of Soursop Leaf Ethanol Extract
This extract is measured for its antioxidant content per 100 grams. The contents measured were phenols, flavonoids, tannins, beta-carotene, vitamin C, tocopherol and saponins (shown in Table 1).
Table 1: Antioxidant content of soursop leaf ethanol extract
|
Antioxidant content (per 100 gram) |
Quantity/Quality (mg) |
|
Phenols |
4876,82 |
|
Flavonoids |
847,23 |
|
Tannins |
20822,09 |
|
Beta-carotene |
4,1556 |
|
Vitamin C |
30402,1 |
|
Tocopherol |
39850,7 |
|
Saponins |
+ |
The results of measuring antioxidant capacity showed very strong results, namely antioxidant capacity of 24,228.60 milligrams of gallic acid per liter of solution (24,228.60 mg/L GAEAC). IC50 measurement results show moderate effectiveness in inhibiting certain targets, namely 32.6230 ppm. In this study, various antioxidant contents were found in the ethanol extract of soursop leaves, namely phenols, flavonoids, tannins, beta-carotene, vitamin C, tocopherol and saponins.
The ethanol extract of soursop leaves per 100 grams showed a very high phenol content. The phenol content in other plants is generally 200-1500 mg 13. The ethanol extract of soursop leaves per 100 grams also shows a very high flavonoid content. The flavonoid content in other plants is generally 50-300 mg 14,15. Tannins in the ethanol extract of soursop leaves in this study were also found in very high levels. In general, the tannin content in plants is 50-3000 mg per 100 grams of plant 14,16,17. The beta-carotene content was found to be quite high in this study. Beta-carotene in other vegetables and fruit is usually 0.4-10 mg per 100 grams 18,19. The results of this research show that the vitamin C content in the ethanol extract of soursop leaves shows extra-ordinary high results. In other plants it is usually only 50-1677 mg per 100 grams 20. The tocopherol content in soursop leaf extract was also found to be extraordinarily high. In other plants it is usually only 20-150 mg per 100 grams 21,22,23. In this study, qualitative saponin content was also found in soursop leaf extract.
Molecular Layer Morphology (Outermost Layer)
In the molecular layer, nerve fiber density was assessed visually by comparing preparations in the control and treatment groups. The scoring system uses a value range of 0-3 with a value of 0 (normal) if the change that occurs is <25%, a value of 1 (mild) if the change that occurs is 25-50%, a value of 2 (moderate) if the change that occurs is 50-75%, and a value of 3 (severe) if the changes that occur are > 75%.
In this study, a significant difference was obtained in the density of nerve fibers (dendrites) between the K- and K+ groups (p=0.000) and P (p=0.015). In addition, a significant difference was obtained between the K+ and P groups (p=0.015), as seen in Figure 1 and 2, and Table 2.
 |
Figure 1: Density of nerve fibers in the molecular layer
|
Table 2: Density of nerve fibers in the molecular layer
|
Density of nerve fibers in the molecular layer |
Frequency and percentage in each group |
||
|
|
K- |
K+ |
P |
|
0% |
4 (26.7%) |
0 (0%) |
1 (6.6%) |
|
25% |
5 (33%) |
0 (0%) |
3 (20%) |
|
50% |
6 (40%) |
4 (26.7%) |
6 (40%) |
|
75% |
0 (0%) |
4 (26.7%) |
4 (26.7%) |
|
100% |
0 (0%) |
7 (46.7%) |
1 (6.6%) |
In group K-, nerve fiber density was found to appear normal to mild changes. In the K+ group, there was a change in nerve fiber density from mild to severe. While in the P group, the density of nerve fibers was from normal to moderate, only 1 rat experienced a severe change in nerve fiber density. In the K- group preparation, the molecular layer of the cerebellum showed that the nerve fibers appeared dense and neatly structured. In K+, there was a decrease in the number of nerve fibers, with areas that appeared emptier or “sparse”. In the P group, the decrease in nerve fibers was not clearly visible, the nerve fibers appeared denser and more neatly structured compared to the K+ group, as seen in Figure 2.
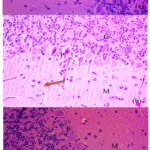 |
Figure 2: The layers of the cerebellum, namely the molecular layer (M), the Purkinje layer (P) and the granular layer (G). a. Control group K-. b. Control group K+, nerve fiber density decreases (orange arrow). c. Treatment group P
|
Purkinje Layer Morphology (Middle Layer)
In the Purkinje layer, Purkinje cell degeneration were assessed. The scoring system uses a value range of 0-3 with a value of 0 (normal) if the change that occurs is <25%, a value of 1 (mild) if the change that occurs is 25-50%, a value of 2 (moderate) if the change that occurs is 50-75%, and a value of 3 (severe) if the changes that occur are > 75%.
To assess Purkinje cell degeneration, an assessment is made on nuclear changes such as karyorrhexis (nuclear fragmentation) or pyknosis (nuclear shrinkage). In addition, an assessment is made on signs of necrosis such as cell swelling, cell membrane disintegration, cell content leakage, and cells that appear blurry and less structured.
In this study, a significant difference was obtained in Purkinje cell degeneration between the K- and K+ groups (p=0.000) and P (p=0.003). In addition, a significant difference was obtained between the K+ and P groups (p=0.015), as seen in Figure 3 and Table 3.
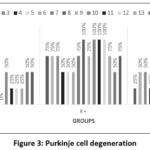 |
Figure 3: Purkinje cell degeneration
|
Table 3: Purkinje cell degeneration
|
Purkinje cell degeneration |
Frequency and percentage in each group |
||
|
|
K- |
K+ |
P |
|
0% |
5 (33%) |
0 (0%) |
0 (0%) |
|
25% |
5 (33%) |
0 (0%) |
4 (26.7%) |
|
50% |
5 (33%) |
4 (26.7%) |
6 (40%) |
|
75% |
0 (0%) |
7 (46.7%) |
3 (20%) |
|
100% |
0 (0%) |
4 (26.7%) |
2 (13%) |
In the K- group, only four out of 15 rats experienced degeneration in Purkinje cells, from mild to moderate. In the K+ group, most samples experienced moderate to severe Purkinje cell degeneration. In the P group, most samples experienced mild to moderate stage, only two rats experienced severe degeneration. The differences in each group can be seen in Figure 2 and 4.
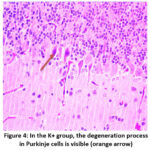 |
Figure 4: In the K+ group, the degeneration process in Purkinje cells is visible (orange arrow).
|
Granular layer morphology (innermost layer)
In the granular layer, granular cell degeneration were assessed. The scoring system uses a value range of 0-3 with a value of 0 (normal) if the change that occurs is <25%, a value of 1 (mild) if the change that occurs is 25-50%, a value of 2 (moderate) if the change that occurs is 50-75%, and a value of 3 (severe) if the changes that occur are > 75%.
Granular cell degeneration is assessed by looking for signs of degeneration such as karyorrhexis (nuclear fragmentation) or pyknosis (nuclear shrinkage). In addition, an assessment is made on signs of necrosis such as cell swelling, cell membrane disintegration, cell content leakage, and cells that appear blurry and less structured.
In this study, there was no significant difference in granular cell degeneration between the K- and K+ groups (p=0.061) and P (p=0.838), as well as between K+ and P (p=0.094) Figures 5 and Table 4.
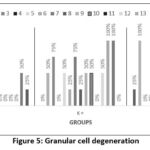 |
Figure 5. Granular cell degeneration
|
Table 4: Granular cell degeneration
|
Granular cell degeneration |
Frequency and percentage in each group |
||
|
|
K- |
K+ |
P |
|
0% |
9 (60%) |
4 (26.7%) |
8 (53%) |
|
25% |
1 (6.6%) |
2 (13%) |
3 (20%) |
|
50% |
4 (26.7%) |
5 (33%) |
2 (13%) |
|
75% |
1 (6.6%) |
2 (13%) |
0 (0%) |
|
100% |
0 (0%) |
2 (13%) |
2 (13%) |
In the K- group, only six out of 15 rats experienced granular cell degeneration, from mild to moderate. In the K+ group, most samples experienced granular cell degeneration from moderate to severe. In the P group, granular cell degeneration occurred at a mild to moderate stage, only two rats experienced severe degeneration. The differences in each group can be seen in Figures 5 and 6.
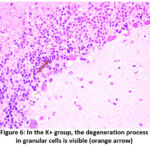 |
Figure 6: In the K+ group, the degeneration process in granular cells is visible (orange arrow)
|
This study showed that noise exposure caused a decrease in nerve fiber density in the K+ group. The group receiving soursop leaf extract (P) showed a non-significant decrease in nerve fiber density compared to the group not receiving the extract, which was seen from the significant difference in the K+ and P groups in nerve fiber density. The density of nerve fibers in the molecular layer of the cerebellum indicates the density or number of synaptic connections in the layer, especially between granular cell axons and Purkinje cell dendrites. The molecular layer serves as a major signal integration area, where granular cell nerve fibers meet Purkinje cell dendrites and climbing fibers from the inferior olivary nucleus. Higher density indicates increased synaptic connectivity and neural activity in the process of motor coordination and motor learning. However, if the density of nerve fibers in this molecular layer decreases, it indicates nerve damage or degeneration, which is likely caused by exposure to various factors such as oxidative stress, noise, or other neurotoxic agents. This can lead to impaired motor function and balance, because disrupted synaptic connectivity will affect signal integration in the cerebellum 24,25.
The results of this study also showed more severe degeneration in Purkinje cells and granular cells in the group that did not receive soursop leaf extract. Degeneration of cerebellar Purkinje cells indicates damage or loss of the main cells responsible for regulating cerebellar output and motor coordination. Purkinje cells are very large neurons with extensively branched dendrites, which receive input from granular cell fibers and climbing fibers from the inferior olivary nucleus, which control signals that exit the cerebellum to other brain structures. If Purkinje cells degenerate, it can cause disturbances in motor coordination and balance, especially fine and coordinated movements, decreased motor learning capacity through plasticity mechanisms, and dysfunction in signal processing and integration, because Purkinje cells are the main signal receivers in the cerebellum. Degeneration of Purkinje cells can be caused by various conditions such as exposure to toxins, oxidative stress, infections and neurodegenerative diseases, and genetics 26,27.
Continuous noise exposure can trigger oxidative stress. Oxidative stress is a condition in which there is an imbalance between the production of free radicals and the body’s ability to neutralize them. This oxidative stress can damage cell membranes, proteins, and DNA, which can then affect nerve cell function. High noise can increase free radical production. This contributes to nerve cell death through apoptosis (programmed cell death) and necrosis (unprogrammed cell death) 28. Cells in the cerebellum layer are particularly susceptible to damage from oxidative stress. Previous research on Wistar rats showed that exposure to high levels of noise can cause morphological damage to several brain areas, which can lead to impaired motor coordination and balance. 29.
Excessive exposure to noise will cause the body to respond by releasing stress hormones such as cortisol and adrenaline. This process can increase the production of free radicals, which are reactive molecules that can damage cells. Free radicals are atoms or molecules that have unpaired electrons, making them highly reactive and can damage cellular components. Increased free radicals can occur through several mechanisms, such as inflammation and mitochondrial dysfunction. Increased stress hormones such as cortisol can affect the immune system and increase the production of pro-inflammatory cytokines. Immune cells that are activated in the inflammatory process can produce free radicals as part of the body’s defense response 30.
Noise can also affect the function of mitochondria, which act as the main source of energy production in cells. When mitochondria are compromised, they produce more free radicals as a byproduct of energy metabolism. Excessive ROS will disrupt mitochondrial function, including ATP production and regulation of cell metabolism 31. Previous research in mice showed that chronic inflammation due to noise can also cause mitochondrial dysfunction. Mitochondrial disorders can also reduce the activity of antioxidant enzymes, such as superoxide dismutase (SOD). With the reduction of these enzymes, the ability of cells to neutralize ROS will decrease, thereby increasing the accumulation of free radicals 32.
This study showed that rats that received soursop leaf extract protection before being exposed to noise experienced a smaller decrease in nerve fiber density compared to rats that did not receive extract protection. The degeneration process in Purkinje cells and granular cells was also lower in rats that received soursop leaf extract protection. This protection can be mediated by phenols, flavonoids, tannins, beta-carotene, vitamin C, tocopherol, saponins contained in this soursop leaf extract.
Soursop leaves are part of the soursop tree plant (Annona muricata L.), growing in tropical regions such as Southeast Asian countries. Soursop leaves are widely used in traditional medicine by the community. The contents of soursop leaf extract such as alkaloids, phenols, flavonoids, tannins, saponins and acetogenin provide health benefits such as anticancer, antimicrobial, antioxidant and anti-inflammatory 17,33,34,35,36.
Phenol is a strong antioxidant and can neutralize free radicals thereby reducing oxidative stress in the body 13. Phenol can also reduce inflammation in the body. Phenolic compounds are absorbed mainly in the small intestine, but their bioavailability varies due to metabolism by intestinal enzymes and gut microbiota. After metabolism, they are excreted in the urine, and to a lesser extent in the bile and feces 37.
Like phenols, flavonoids are also compounds that have strong antioxidant properties so they can neutralize free radicals and reduce oxidative stress in the body. Flavonoids can also inhibit enzymes and molecular pathways that play a role in the inflammatory process 14,16,17. Flavonoids are metabolized in the liver and intestine, with limited absorption in the small intestine. Some flavonoids are further metabolized by the gut microbiota to produce more absorbable compounds. Flavonoids and their metabolites are excreted primarily in the urine. Some flavonoids are also excreted in the bile and excreted in the feces 38.
The tannins contained in this extract also have the same benefits as phenols and flavonoids 14,16,17. Tannin compounds are large and complex in size, so that little can be absorbed and most of them undergo hydrolysis in the intestine by microbiota, then producing simpler phenolic compounds that can be absorbed. Excretion of metabolite products from tannins is generally excreted through urine and feces 39.
Beta-carotene also has strong antioxidant capabilities so it can also protect the body from oxidative stress 40. Beta-carotene is a precursor of vitamin A and is absorbed in the small intestine with the help of dietary fat. After being converted to vitamin A in the liver, some beta-carotene is stored in fat tissue. Beta-carotene is excreted mainly in the feces, mainly in unabsorbed form, while vitamin A is excreted in the urine 41.
Vitamin C is also a strong antioxidant so it can protect cells from damage caused by free radicals 20. Vitamin C can be absorbed in the small intestine through active transport and passive diffusion. Its bioavailability is quite high until it reaches saturation, after which its excretion increases. Unused vitamin C is excreted in the urine. Excretion of this vitamin can increase as the concentration in plasma increases beyond the body’s needs 42.
Tocopherol provides health benefits because it is also a strong antioxidant 21,22,23. Tocopherol (vitamin E) is fat-soluble and can be absorbed in the small intestine with dietary fat, then transported in the lymphatic system. The absorption process involves micelles and lipoproteins. This vitamin is excreted mainly through bile and then into the feces. A small portion of the metabolite products of this vitamin can also be excreted through urine 43,44.
Saponins are also beneficial for health. Saponin also acts as an antioxidant and can inhibit cancer proliferation and trigger apoptosis 45. Lastly, saponins can reduce inflammation because they can inhibit inflammatory mediators such as prostaglandins and inflammatory cytokines 46. Saponins are generally poorly absorbed due to their large and complex molecules, but some types of saponins can be hydrolyzed by intestinal enzymes or microbiota into more easily absorbed aglycones. Unabsorbed saponins and their metabolites are excreted primarily through feces 47.
Based on previous research, it was found that soursop leaf extract can help protect body cells from oxidative damage caused by free radicals due to the high phenolic content of this extract 17. Previous in vitro research using lipopolysaccharide-induced cells showed that soursop leaf extract also had anti-inflammatory effects on macrophages stimulated by lipopolysaccharide. This is supported by the results of other literature studies 16,35,48.
The antioxidant capacity of the ethanol extract of soursop leaves is also very high. In other plants, several hundred to 20,000 mg/L GAEAC is usually obtained. This of course supports high protection against free radicals which contribute to aging and the development of various chronic diseases. High antioxidant capacity can also reduce the risk of heart disease, prevent cancer, and strengthen immunity 49,50,51,52,53.
This research shows that the ethanol extract of soursop leaves has a fairly good IC50. IC50 (Inhibitory Concentration 50) is a measurement used as an indicator of the effectiveness of a compound in inhibiting certain biological or biochemical processes. Herbal studies show that if a compound has an IC50 between 20-50 ppm (parts per million) it is categorized as having moderate to high antioxidant effectiveness. With an IC50 of 32.6230 ppm, it shows that the compounds in this extract have a good ability to inhibit free radicals and help protect cells from oxidative damage. Apart from that, it can help improve cell health by reducing oxidative stress and inflammation which contribute to aging and various degenerative diseases. It can be concluded that the compounds in this extract have good antioxidant potential for use in the formulation of antioxidant supplements, skin care products, and drug development for diseases related to oxidative stress 46,53,54,55.
Previous research shows that soursop leaf extract has a protective effect against oxidative damage to neurons 56. Another study on rats that experienced nerve injury due to radiation, also found that soursop leaf extract could reduce inflammation in the brain, which is the main risk factor for neuron damage. This shows that soursop leaf extract can maintain the integrity of neurons in the brain, including the cerebellum 57.
Soursop leaf extract has neuroprotective potential, because this extract contains bioactive compounds that can protect neurons from oxidative stress, including inflammation 56. Soursop leaf extract has the ability to neutralize free radicals and reduce inflammation at the cellular level, thereby having a positive effect on the health of neurons, including the cerebellum 58.
The content of anonaceous acetogenins can influence various cellular signaling pathways, such as activation of the PI3K/Akt pathway which plays an important role in the regulation of cell survival, cell growth and metabolism. Activation of this pathway will protect neurons from apoptosis and oxidative stress 59. Another signaling pathway that can be influenced is by modulating the NF-κB pathway involved in inflammatory responses and cell survival. Apart from that, the content of acetogenin, alkaloids and annonaceous flavonoids contained in this extract can also influence the Mitogen-Activated Protein Kinase/Extracellular Signal-Regulated Kinase (MAPK/ERK) pathway which plays a role in cell proliferation, differentiation and response to stress, so that contributes to neuroprotective effects by reducing apoptosis and increasing neuronal survival 56,60.
The flavonoid content such as quercetin and kaempferol in soursop leaf extract has a strong antioxidant effect so it can reduce oxidative stress. Kaempferol can also inhibit NF-κB which plays a role in inflammatory responses and apoptosis. By inhibiting NF-κB activity, it will reduce the expression of pro-inflammatory genes and cytokines, thereby reducing inflammation and protecting neurons from damage due to inflammation 61. The flavonoid mechanism for reducing oxidative stress is by activating the Nrf2 pathway which is able to regulate the expression of antioxidant enzymes 62. Research in mice shows that flavonoids have neuroprotective abilities from neurotoxins by increasing dopamine and serotonin levels in the striatum, inhibiting oxidative stress, and the response of astroglia 63.
Soursop leaf extract also contains saponins which have anti-inflammatory and antioxidant effects. Saponins can neutralize free radicals, thereby helping protect neurons from damage and apoptosis. Saponins are also able to inhibit inflammatory pathways such as NF-κB and reduce the production of pro-inflammatory cytokines, thereby reducing inflammation in neurons 64. Previous in vitro studies have shown that saponins have neuroprotective effects on spinal cord neurons 65.
Beta-carotene is a type of carotenoid, also contained in this extract. Beta-carotene can neutralize free radicals and oxidative stress in neurons, thereby preventing damage to cell membranes, proteins and DNA in neurons. Beta-carotene is also a precursor of vitamin A which is important for the normal function of the nervous system, where this vitamin plays a role in the regulation of gene expression related to the growth and development of neurons 66. Previous studies in rats have shown that beta-carotene reduces the progression of nerve injury through inhibition of NF-κB activation 67.
The ability of soursop leaf extract to provide neuroprotective effects can be linked to its ability to penetrate the blood brain barrier (BBB). However, research on the ability of soursop leaf extract to penetrate the BBB is still limited. There are several factors in this extract that can affect its ability to penetrate the BBB, namely bioactive components and molecular size. Smaller and lipophilic compounds contained in the extract such as acetogenins, flavonoids, tannins, and phenols, have a more active transport mechanism, so they are more likely to penetrate the BBB. In addition, other factors such as compounds require metabolism first to become smaller molecules to be able to penetrate the BBB. This shows that not all compounds in this extract are able to penetrate the BBB, but there is a mechanism of compounds in this extract that can produce neuroprotective effects without having to penetrate directly into the BBB, namely its systemic effects through reducing oxidative stress and inflammation throughout the body, stimulation of signaling pathways in the periphery that can affect brain function, and through metabolites that can penetrate the BBB 68,69,70.
Conclusion
Ethanol extract of soursop leaves can provide neuroprotection against chronic noise exposure. In this study, it was proven that the content of ethanol extract of soursop leaves in the form of phenols, flavonoids, tannins, beta-carotene, vitamin C, tocopherol, and saponins with moderate to high antioxidant effectiveness can provide protection for neurons in the cerebellum of rats exposed to noise.
Acknowledgment
The author would like to thank the laboratory team who were very persistent in helping the author during the implementation at the integrated laboratory at Universitas Udayana, as well as to all participants involved in completing this research.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article
Conflict of Interest
The author(s) do not have any conflict of interest
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
The research protocol has been approved by the Research Ethics Committee, Faculty of Medicine, Udayana University, Denpasar, Indonesia with approval number 1072/UN14.2.2.VII.14/LT/2024 for phytochemical testing of soursop leaf extract, and approval number 1397/UN14.2.2.VII.14/LT/2024 for research with experimental animals.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required
Clinical Trial Registration
This research does not involve any clinical trials
Author Contributions
Dewa Ayu Agung Alit Suka Astini: Conceptualization, Methodology, Writing – Original Draft.
I Wayan Putu Sutirta Yasa: Acquisition, Resources, Supervision.
I Made Jawi: Acquisition, Resources, Supervision.
I Nyoman Wande: Writing – Review & Editing.
Putu Indah Budi Apsari: Writing – Review & Editing.
Luh Gde Evayanti: Data Collection, Analysis.
References
- Ilango S, Sahoo DK, Paital B, Kathirvel K, Gabriel JI, Subramaniam K, Jayachandran P, Dash RK, Hati AK, Behera TR, Mishra P, Nirmaladevi R. A Review on Annona muricata and Its Anticancer Activity. Cancers (Basel). 2022;14(18):1-31.
CrossRef - Olugbuyiro JA, Omotosho OE, Taiwo OS, Ononiwu F O, Banwo AS, Akintokun OA, Obaseki OS, Ogunlenye OM. Antimicrobial Activities and Phytochemical Properties of Annona muricata Leaf. Covenant J Phys Life Sci. 2017;5(2):40-49.
- Morayo Ale E, Adeleye AO, Akinseye OR, Toluwalase EK. Antioxidant activities of ethanolic extract of Annona muricata against different pro-oxidant induced lipid peroxidation in rat brain and liver. Pharm Pharmacol Int J. 2021;9(2):45-49.
CrossRef - Huang L, Zhang Y, Wang Y, Lan Y. Relationship between Chronic Noise Exposure, Cognitive Impairment, and Degenerative Dementia: Update on the Experimental and Epidemiological Evidence and Prospects for Further Research. J Alzheimer’s Dis. 2021;79(4):1409-1427.
CrossRef - Cui B, Su D, Li W, She X, Zhang M, Wang R, Zhai Q. Effects of chronic noise exposure on the microbiome-gut-brain axis in senescence-accelerated prone mice: Implications for Alzheimer’s disease. J Neuroinflammation. 2018;15(1):1-15.
CrossRef - Alarif WM, Al-Lihaibi SS, Bawakid NO, Abdel-Lateff A, Al-malky HS. Rare acetogenins with anti-inflammatory effect from the red alga laurencia obtusa. Molecules. 2019;24(3).
CrossRef - Martínez-Coria H, Arrieta-Cruz I, Gutiérrez-Juárez R, López-Valdés HE. Anti-Inflammatory Effects of Flavonoids in Common Neurological Disorders Associated with Aging. Int J Mol Sci. 2023;24(5).
CrossRef - Wurdianing I, Nugraheni S, Rahfiludin Z. Efek ekstrak daun sirsak (Annona muricata Linn) terhadap profil lipid tikus putih jantan (Rattus Norvegicus). J Gizi Indones (The Indones J Nutr. 2014;3(1):7-12.
CrossRef - Fadel MN, Besan EJ. UJI AKTIVITAS ANTIDIABETES EKSTRAK DAUN SIRSAK (Annona muricata L.) PADA MENCIT YANG DIINDUKSI ALOKSAN. Indones J Farm. 2021;5(2):1.
CrossRef - Adewole S, Caxton-Martins E. Morphological changes and hypoglycemic effects of Annona muricata linn. (annonaceae) leaf aqueous extract on pancreatic β-cells of streptozotocin-treated diabetic rats. African J Biomed Res. 2009;9(3):173-187.
CrossRef - Zubaidi SN, Qadi WSM, Maarof S, Misnan NM, Noor HSM, Hamezah HS, Baharum SN, Rosli N, Jam FA, Al-Olayan E, Wang C, Hellal K, Buzgaia N, Mediani A. Assessing the Acute Toxicological Effects of Annona muricata Leaf Ethanol Extract on Rats: Biochemical, Histopathological, and Metabolomics Analyses. toxics. Published online 2023.
CrossRef - Utomo AW, Susilaningsih N, Armalina D. Acute Toxicity Test of Soursop Leaves (Annona muricata) on Liver and Kidney of Switzerland Mice. Sains Med J Kedokt dan Kesehat. 2016;6(2):48.
CrossRef - Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006;99(1):191-203.
CrossRef - Khurana S, Venkataraman K, Hollingsworth A, Piche M, Tai TC. Polyphenols: Benefits to the cardiovascular system in health and in aging. Nutrients. 2013;5(10):3779-3827.
CrossRef - Middleton E, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52(4):673-751.
- Gavamukulya Y, Wamunyokoli F, El-Shemy HA. Annona muricata: Is the natural therapy to most disease conditions including cancer growing in our backyard? A systematic review of its research history and future prospects. Asian Pac J Trop Med. 2017;10(9):835-848.
CrossRef - Moghadamtousi SZ, Fadaeinasab M, Nikzad S, Mohan G, Ali HM, Kadir HA. Annona muricata (Annonaceae): A review of its traditional uses, isolated acetogenins and biological activities. Int J Mol Sci. 2015;16(7):15625-15658.
CrossRef - Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr. 2003;23(February 2003):171-201.
CrossRef - Marzęda P, Łuszczki J. Role of vitamin A in health and illness. J Pre-Clinical Clin Res. 2019;13(3):137-142.
CrossRef - Padayatty SJ, Levine M. Vitamin C : the known and the unknown and Goldilocks. Oral Dis. Published online 2016:463-493.
CrossRef - Meydani SN, Meydani M, Blumberg JB, Leka LS, Siber G, Loszewski R, Thompson C, Pedrosa MC, Diamond RD, Stollar BD. Vitamin E Supplementation and In Vivo Immune Response in Healthy Elderly Subjects. Am J Clin Nutr. Published online 1997.
CrossRef - Placzek M, Gaube S, Kerkmann U, Gilbertz KP, Herzinger T, Ekkehard H, Przybilla B. Ultraviolet B-Induced DNA Damage in Human Epidermis Is Modified by the Antioxidants Ascorbic Acid and D – a -Tocopherol. J Invest Dermatol. 2004;2:304-307.
CrossRef - Traber MG, Atkinson J. Vitamin E , antioxidant and nothing more. Free Radic Biol Med. 2007;43:4-15.
CrossRef - Kim J, Augustine GJ. Molecular Layer Interneurons: Key Elements of Cerebellar Network Computation and Behavior. Neuroscience. 2021;462:22-35.
CrossRef - Osório C, Watt AJ, Kisiswa L. Editorial: Molecular mechanisms and pathways in cerebellar function. Front Mol Neurosci. 2023;16.
CrossRef - Cook AA, Fields E, Watt AJ. Losing the Beat: Contribution of Purkinje Cell Firing Dysfunction to Disease, and Its Reversal. Neuroscience. 2021;462:247-261.
CrossRef - Fleming JT, He W, Hao C, Ketova T, Pan FC, Wright CC V, Litingtung Y, Chiang C. The Purkinje neuron acts as a central regulator of spatially and functionally distinct cerebellar precursors. Dev Cell. 2014;27(3).
CrossRef - Gröschel M, Basta D, Ernst A, Mazurek B, Szczepek AJ. Acute noise exposure is associated with intrinsic apoptosis in murine central auditory pathway. Front Neurosci. 2018;12(MAY):1-14.
CrossRef - Frenzilli G, Ryskalin L, Ferrucci M, Cantafora E, Chelazzi S, Giorgi FS, Lenzi P, Scarcelli V, Frati A, Biagioni F, Gambardella S, Falleni A, Fornai F. Loud noise exposure produces DNA, neurotransmitter and morphological damage within specific brain areas. Front Neuroanat. 2017;11(June):1-16.
CrossRef - Hahad O, Prochaska JH, Daiber A, Muenzel T. Environmental Noise-Induced Effects on Stress Hormones, Oxidative Stress, and Vascular Dysfunction: Key Factors in the Relationship between Cerebrocardiovascular and Psychological Disorders. Oxid Med Cell Longev. 2019;2019.
CrossRef - Yang ZJ. ROS-induced oxidative stress and mitochondrial dysfunction: a possible mechanism responsible for noise-induced ribbon synaptic damage. Am J Transl Res. 2024;16(1):272-284.
CrossRef - Zhang L, Du Z, He L, Liang W, Liu K, Gong S. ROS-Induced Oxidative Damage and Mitochondrial Dysfunction Mediated by Inhibition of SIRT3 in Cultured Cochlear Cells. Neural Plast. 2022;2022.
CrossRef - Nwokocha CR, Owu DU, Gordon A, Thaxter K, Mccalla G, Ozolua RI, Young L. Possible mechanisms of action of the hypotensive effect of Annona muricata (soursop) in normotensive SpragueDawley rats. Pharm Biol. 2012;50(11):1436-1441.
CrossRef - Hamizah S, Roslida AH, Fezah O, Tan KL, Tor YS, Tan CI. Chemopreventive potential of Annona muricata L leaves on chemically-induced skin papillomagenesis in mice. Asian Pacific J Cancer Prev. 2012;13(6):2533-2539.
CrossRef - Shin YM, Kim YJ. Anti-inflammatory effects of ethanolic extract of Annona muricata . J Cosmet Med. 2023;7(1):25-28.
CrossRef - Torres MP, Rachagani S, Purohit V, Pandey P, Joshi S, Moore ED, Johansson SL, Singh PK, Ganti AK, Batra SK. Graviola: A Novel Promising Natural-Derived Drug That Inhibits Tumorigenicity and Metastasis of Pancreatic Cancer Cells In Vitro and In Vivo Through Altering Cell Metabolism. Cancer Lett. 2004;33(3):97.
- Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81(1 Suppl).
CrossRef - Hollman PCH. Absorption, bioavailability, and metabolism of flavonoids. Pharm Biol. 2004;42(SUPPL.):74-83.
CrossRef - Hagerman AE, Robbins CT, Weerasuriya Y, Wilson TC, McArthur C. Tannin Chemistry in Relation to Digestion. J Range Manag. 1992;45(1):57.
CrossRef - Maiani G, Castón MJP, Catasta G, Toti E, Cambrodón IG, Bysted A, Granado-Lorencio F, Olmedilla-Alonso B, Knuthsen P, Valoti M, Böhm V, Mayer-Miebach E, Behsnilian D, Schlemmer U. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol Nutr Food Res. 2009;53(SUPPL. 2):194-218.
CrossRef - Harrison EH. Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. Biochim Biophys Acta. 2009;61(1):1-7.
- Lykkesfeldt J, Tveden-Nyborg P. The pharmacokinetics of vitamin C. Nutrients. 2019;11(10).
CrossRef - Anwar K, Iqbal J, Hussain MM. Mechanisms involved in vitamin e transport by primary enterocytes and in vivo absorption. J Lipid Res. 2007;48(9):2028-2038.
CrossRef - Rigotti A. Absorption, transport, and tissue delivery of vitamin E. Mol Aspects Med. 2007;28(5-6):423-436.
CrossRef - Man S, Gao W, Zhang Y, Huang L, Liu C. Fitoterapia Chemical study and medical application of saponins as anti-cancer agents. Fitoterapia. 2010;81(7):703-714.
CrossRef - Kim JY, Wang YPH, Im DHK, An EHH, Hung YCC, Oh SHR, Eong HGJ. Inhibitory Effect of the Saponins Derived from Roots of Platycodon grandiflorum on Carrageenan-Induced Inflammation. Biosci Biotechnol Biochem. 2006;70(4):858-864.
CrossRef - Timilsena YP, Phosanam A, Stockmann R. Perspectives on Saponins: Food Functionality and Applications. Int J Mol Sci. 2023;24(17).
CrossRef - Cushnie TPT, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005;26(5):343-356.
CrossRef - Stansfeld SA, Matheson MP. Noise pollution: Non-auditory effects on health. Br Med Bull. 2003;68(February 2003):243-257.
CrossRef - Dixit M, Vidyapeeth TM, Pandit A, Vidyapeeth TM. Role of Antioxidants in the Prevention of Cancer : A Comprehensive Review Role of Antioxidants in the Prevention of Cancer : A Comprehensive Review. UGC CARE J. 2023;48(2):1318-1329.
- Kris-etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, Griel AE, Etherton TD. Bioactive Compounds in Foods : Their Role in the Prevention of Cardiovascular Disease and Cancer. Am J Med. 2002;113(9):71-88.
CrossRef - Rahman K. Studies on free radicals , antioxidants , and co-factors. Clin Interv Aging. 2007;2(2):219-236.
- Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44-84.
CrossRef - Floegel A, Kim D ok, Chung S jin, Koo SI, Chun OK. Journal of Food Composition and Analysis Comparison of ABTS / DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J Food Compos Anal. 2011;24(7):1043-1048.
CrossRef - Tava A, Avato P. Natural Product Communications Chemical and Biological Activity of Triterpene Saponins from Medicago Species. Nat Prod Commun. 2006;1(12):1159-1180.
CrossRef - Kim WS, Kim YE, Cho EJ, Byun EB, Park WY, Song HY, Kim K, Park SH, Byun EH. Neuroprotective effect of Annona muricata-derived polysaccharides in neuronal HT22 cell damage induced by hydrogen peroxide. Biosci Biotechnol Biochem. 2020;84(5):1001-1012.
CrossRef - Elmas O, Keskin E, Keser Sahin HH, Guven B, Almisned G, Zakaly HMH, Tekin HO, Ene A. The effect of Annona muricata (Graviola) on the prevention of brain damage due to ionizing radiation in rats. Heliyon. 2024;10(4):e25932.
CrossRef - Arnaud K, Nicodème C, Durand DN, Martial N, Basile S, Haziz S, Christine N, Christian KA, Halfane L, Victorien D, Noumavo P, Lamine BM. Antioxidant, Anti-Inflammatory Efficacy and HPLC Analysis of Annona muricata Leaves Extracts from Republic of Benin. Am J Plant Sci. 2020;11(06):803-818.
CrossRef - Mittal R, Chaudhry N, Mukherjee TK. Targeting breast cancer cell signaling molecules PI3K and Akt by phytochemicals Cannabidiol, Nimbin and Acetogenin: An in silico approach . J Biomed. 2018;3(4):60-63.
CrossRef - Wahab SMA, Jantan I, Haque MA, Arshad L. Exploring the leaves of Annona muricata L. as a source of potential anti-inflammatory and anticancer agents. Front Pharmacol. 2018;9(JUN):1-20.
CrossRef - Ye Y, Zhou J. The protective activity of natural flavonoids against osteoarthritis by targeting NF-κB signaling pathway. Front Endocrinol (Lausanne). 2023;14(March):1-17.
CrossRef - Xu W, Lu H, Yuan Y, Deng Z, Zheng L, Li H. The Antioxidant and Anti-Inflammatory Effects of Flavonoids from Propolis via Nrf2 and NF-κB Pathways. Foods. 2022;11(16):1-36.
CrossRef - Cheng Y, He G, Mu X, Zhang T, Li X, Hu J, Xu B, Du G. Neuroprotective effect of baicalein against MPTP neurotoxicity: Behavioral, biochemical and immunohistochemical profile. Neurosci Lett. 2008;441(1):16-20.
CrossRef - Khan MI, Karima G, Khan MZ, Shin JH, Kim JD. Therapeutic Effects of Saponins for the Prevention and Treatment of Cancer by Ameliorating Inflammation and Angiogenesis and Inducing Antioxidant and Apoptotic Effects in Human Cells. Int J Mol Sci. 2022;23(18).
CrossRef - Liao B, Newmark H, Zhou R. Neuroprotective effects of ginseng total saponin and ginsenosides Rb1 and Rg1 on spinal cord neurons in Vitro. Exp Neurol. 2002;173(2):224-234.
CrossRef - Marie A, Darricau M, Touyarot K, Parr-Brownlie LC, Bosch-Bouju C. Role and Mechanism of Vitamin A Metabolism in the Pathophysiology of Parkinson’s Disease. J Parkinsons Dis. 2021;11(3):949-970.
CrossRef - Zhou L, Ouyang L, Lin S, Chen S, Liu YJ, Zhou W, Wang X. Protective role of β-carotene against oxidative stress and neuroinflammation in a rat model of spinal cord injury. Int Immunopharmacol. 2018;61(February):92-99.
CrossRef - Youdim KA, Dobbie MS, Kuhnle G, Proteggente AR, Abbott NJ, Rice-Evans C. Interaction between flavonoids and the blood-brain barrier: In vitro studies. J Neurochem. 2003;85(1):180-192.
CrossRef - Sweeney MD, Sagare AP, Zlokovic B V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14(3):133-150.
CrossRef - Lobine D, Sadeer N, Jugreet S, Suroowan S, Keenoo BS, Imran M, Venugopala KN, Ibrahim FM, Zengin G, Mahomoodally MF. Potential of Medicinal Plants as Neuroprotective and Therapeutic Properties Against Amyloid-β-Related Toxicity, and Glutamate-Induced Excitotoxicity in Human Neural Cells. Curr Neuropharmacol. 2021;19(9):1416-1441.
CrossRef








