Poovizhi Bharathi Rajaduraivelpandian , Shamitha Rai
, Shamitha Rai , Rashmi Raghava Rao*
, Rashmi Raghava Rao* , Trishna Sudarshan
, Trishna Sudarshan and Ashitha Leslie Mariam
and Ashitha Leslie Mariam
Department of Pharmacology, Kasturba Medical College, Mangalore, Manipal Academy of Higher Education, Manipal, Karnataka, India.
Corresponding Author E-mail: rashmi.rao@manipal.edu
DOI : https://dx.doi.org/10.13005/bpj/3034
Abstract
Introduction: Dementia is a progressive deterioration in cognitive abilities that hinders one's capacity to function independently. The present dementia treatment includes galantamine, rivastigmine, donepezil ,and memantine. However, they trigger many cardiovascular complications including syncopal episodes and myocardial infarction. Herbal medications are noted for their efficacy and absence of adverse pharmacological consequences. Hence the quest for herb-based medicines is happening. ‘Terminalia bellirica fruit pulp’ alleviates an array of illnesses. Aim: The ‘aqueous extract of Terminalia bellirica fruit pulp’ (AETBFP) was examined for cognitive-enhancing effects on rodents. Methods: Hebbs William and Elevated Plus Maze models were utilized to test the cognitive-enhancing properties of the fruit pulp. Forty-two Wistar rats were grouped into positive control (normal saline), negative control (scopolamine alone), standard (piracetam), and four test groups administered with the fruit extract at doses 143 mg/kg, 200 mg/kg, 334 mg/kg, and 334 mg/kg + Piracetam (600mg/kg) respectively. The animals received treatment for 14 days and on day 14 all the groups were administered scopolamine (1 mg/kg). Results: The four test groups (AETBFP 143 mg/kg, 200 mg/kg, 334 mg/kg, and 334 mg/kg + Piracetam) significantly decreased (p<0.05) the Time to reach the reward chamber in ‘Hebb’s William maze’ and the test groups (AETBFP 200 mg/kg, 334 mg/kg, and 334 mg/kg + Piracetam) significantly decreased (p<0.05) transfer latency in ‘elevated pluz maze’. A significant cognitive enhancing effect was reported with AETBFP which could be attributed to its antioxidant and neuroprotective action.
Keywords
Cognition; Elevated pluz maze; Hebbs William maze; Rats; Scopolamine; Terminalia bellirica
Download this article as:| Copy the following to cite this article: Rajaduraivelpandian P. B, Rai S, Rao R. R, Sudarshan T, Mariam A. L. The Cognitive-Enhancing Properties of the Aqueous Extract from the Fruits of Terminalia bellirica (Gaertn.) Roxb. Biomed Pharmacol J 2024;17(4). |
| Copy the following to cite this URL: Rajaduraivelpandian P. B, Rai S, Rao R. R, Sudarshan T, Mariam A. L. The Cognitive-Enhancing Properties of the Aqueous Extract from the Fruits of Terminalia bellirica (Gaertn.) Roxb. Biomed Pharmacol J 2024;17(4). Available from: https://bit.ly/3W1g4o9 |
Introduction
Dementia is derived from the Latin phrase ‘demens’, which means ‘being out of one’s mind‘.1 The medical term “dementia” represents a clinical condition typified by a progressive decline in cognitive abilities, which inhibits an individual’s ability to operate independently.2 Dementia strikes a person in the globe every three seconds.3 There are around ten million fresh cases every single year. Currently, around fifty-five million people worldwide suffer from cognitive impairment, especially over sixty percent of those inhabiting nations with low to middle incomes.4 There exist multiple types of dementia, such as ‘mixed dementia’, ‘frontotemporal lobar degeneration’, ‘Lewy body dementia’, ‘vascular dementia’, ‘Creutzfeldt-Jakob disease’, and ‘normal pressure hydrocephalus’.5 Alzheimer’s is its most prevalent type.6
The main objective of the present dementia treatment is to activate the NMDA (N-methyl-D-aspartate) receptors and enhance the transmission of cholinergic neurons by using acetylcholinesterase inhibitors (galantamine, rivastigmine, donepezil) and memantine, an antagonist of NMDA receptor.7 Donepezil may trigger syncopal episodes and heart block because of its vagotonic characteristics.8Galantamine increases the risk of QT interval prolongation, while rivastigmine may raise the risk of stroke, torsade de pointes and myocardial infarction. A Higher rate of memantine discontinuation occurs as a result of adverse events.9
There exists a recent unprecedented drive in modern medical practices for the utilization of herb-based remedies due to their multi-target potential and various pharmacological effects.10This impetus arise from a multitude of factors, primary amongst those is the interpretation that herbal medications are safer and cost-effective as compared with current standard therapies.11Therefore, the formulation of novel compounds with a superior pharmacological profile aided by herbal medications is critical.12
The Terminalia bellirica tree, which is a member of the Terminalia Linn. genus and Combretaceae family, yields Terminalia bellirica fruit. Its distribution is primarily in Southeast Asia.13 Bangladesh, India, Cambodia, Bhutan, China, Laos, Indonesia, Malaysia, Pakistan, Nepal, Sri Lanka, Vietnam and Thailand are the native habitats of Terminalia bellerica.14Throughout the Indian subcontinent, Terminalia is frequently found in native woods in Karnataka, West Bengal, Uttar Pradesh, Madhya Pradesh, Maharashtra, Tamil Nadu, Assam, Rajasthan, Punjab and Kerala.15
Roxburgh W, Banks J, Bulmer W, Mackenzie D and Nicol G wrote the first scientific description of Terminalia bellerica.16
Terminalia bellerica is a gigantic deciduous tree up to 50 meters tall and approximately 3 meters in diameter, featuring a round crown. Fruit is broadly ellipsoid to sub-globular (20-40 x 18-22 millimeters), thickly velutinous or sericeous, mild-yellow, seemingly 5-angled, and precisely brown tomentosa. The name ‘Terminalia‘ is derived from the Latin word ‘terminus’ and alludes to the clustering of leaves on the terminals of branches.17
Many traditional medical systems, including Unani, Ayurveda, Siddha, and Chinese traditional medicine, have used Terminalia bellirica.18 Terminalia bellerica possesses components that protect the liver from damage, stimulate the immune system, combat cancer, control diabetes, and have antimicrobial, antihypertensive, antioxidant, and anti-diarrheal properties.19 Terminalia bellerica is used as an astringent, laxative, antipyretic and anthelmintic. Terminalia bellerica fruits are often used as a hair tonic and to treat a variety of conditions, including bronchitis, hepatitis, asthma, piles, dyspepsia, diarrhea, hoarseness of the voice, coughs, eye disorders, and scorpion stings.20The well-known Ayurvedic medicine triphala is a compound made up of the dried fruits of three different plant species: Terminalia bellirica (family- Combretaceae), Terminalia chebula (family- Combretaceae) and Emblica officinalis (family- Euphorbiaceae).21Extract from Terminalia chebula prevents amnesia induced by scopolamine.22Tannoid components found in Emblica officinalis have been observed for restoring High-sodium, high-cholesterol diet and aluminum chloride-induced cognitive deficiencies. 23,24
Consequently, the researchers sought to determine whether the third component of Triphala, Terminalia bellirica fruit pulp possessed characteristics that would enhance cognition. Furthermore, the current study can serve as a reference for future investigations on the molecular process behind the given results.
Materials and Methods
Ethical Consideration
The protocol received Institutional Animal Ethics Committee (IAEC) approval for a three-month research period involving forty-two ‘Wistar albino rats’ (IAEC approval number: KMC.MNG/IAEC/08/2023). The CCSEA registration number assigned to the IAEC is 213/PO/Re/S/2000/CPCSEA07/09/2022 (Valid up to 06/09/2027). The rodents were reared in the Pharmacology department’s animal house, KMC Mangalore, Karnataka, India. The research study was conducted in the Experimental laboratory, Pharmacology department, Kasturba Medical College, Mangalore, India.
Experimental animals
Forty-two Wistar albino male rats weighing 180 to 220 grams and aged between three and five months were housed in hygienic cages made of polypropylene at standardized environmental conditions (Temperature: 22±30°C, Humidity: 50-60%; dark/light cycle: 12 hours.). Rodents were provided with water and a standard diet ad libitum. A one-week acclimatization period was provided to ensure that rodents adapt to the study settings. To reduce disparities, readings were taken in a calm environment between nine in the morning and noon consistently. The study adhered to the guidelines established by the CCSEA (Committee for Control and Supervision of Experiments on Animals).
Study plant material and Drugs
Positive control – Normal saline, Negative control Scopolamine hydrobromide (Sigma-Aldrich) and Standard control- Piracetam (Intas Pharmaceuticals Ltd) were procured from KMC Mangalore Hospital Pharmacy, Karnataka, India. All Terminalia bellerica fruit pulps were obtained from Lakshmi Ayurveda Pharmacy, Mangalore, Karnataka, India.
Authentication
Dr. Jyothi Miranda currently an Associate Professor in the Botany department, St. Aloysius College, Mangalore, Karnataka, India, authenticated the Terminalia bellirica fruit pulps.
Preparation of ‘aqueous extract of Terminalia bellirica fruit pulp’ (AETBFP)
Fruit pulps were rinsed with flowing water and thoroughly dried out in shady conditions. Dried fruit pulps were then finely ground up utilizing a grinder. Soxhlet extraction was used to extract 1,000 grams of raw AETBFP granules which was then air-dried over 36 hours. Rotator evaporation was utilized to dehydrate the powder under regulated pressure at 40-50°C temperature. AETBFP yielded a brownish lump weighing 145 grams. The dehydrated crude powder of extract produced approximately 14.5 percent by weight/weight.
Treatment grouping and dosage
The rats were randomly separated into seven groups, comprising six rats per group (n = 3 males and 3 females) as seen in Table 1, they are housed in different cages. Group one acted as the positive control (0.9% normal saline, 10 ml/kg body weight, oral). Group two served as a negative control (scopolamine, 1 mg/kg body weight, intraperitoneal.). Group three served as a standard control (Piracetam at 600 mg/kg body weight per oral + scopolamine).25 Groups four to six received the test drug (AETBFP 143, 200, and 334 mg /kg body weight per oral + scopolamine) respectively.26 Group seven received AETBFP (334mg/kg body weight per oral) + piracetam and scopolamine. Apart from scopolamine, all other drugs were administered once every day for 14 days. On day fourteen, all the rats received scopolamine (1 mg / kg body weight, intraperitoneal), one hour after administering either the standard drug or the AETBFP to the appropriate groups, except the normal control group.
Table 1: Treatment Groups
| Group
(n=6) |
Treatment received |
| 1 | Positive control – Normal saline (10ml/kg b.w), p.o. for 14 days |
| 2 | Negative control- Scopolamine (1mg/kg b.w), i.p. on 14th day |
| 3 | Standard control- Piracetam (600mg/kg b.w), p.o. for 14 days + Scopolamine (1mg/kg b.w), i.p. on 14th day |
| 4 | Test 1- AETBFP (143mg/kg b.w), p.o. for 14 days + Scopolamine (1mg/kg b.w), i.p. on 14th day |
| 5 | Test 2- AETBFP (200mg/kg b.w), p.o. for 14 days + Scopolamine (1mg/kg b.w), i.p. on 14th day |
| 6 | Test 3-AETBFP (334mg/kg b.w), p.o. for 14 days + Scopolamine (1mg/kg b.w), i.p. on 14th day |
| 7 | AETBFP (334mg/kg b.w), i.p. for 14 days + Piracetam (600 mg/kg b.w), p.o. for 14 days + Scopolamine (1mg/kg b.w ), i.p. on 14th day |
p.o. – Per oral, i.p.- Intraperitoneal, b.w- body weight, AETBFP- ‘aqueous extract of Terminalia bellirica fruit pulp
Experimental procedure
On day 14, cognitive paradigms were assessed 45 minutes after the scopolamine injection using the Hebb-William Maze and the elevated plus maze. This is referred to as the ‘acquisition trial (AT)’ and is related to learning. Furthermore, on day 15 shortly after 24 hours of the scopolamine injection, a ‘retention trial (RT)’ was performed to evaluate the index for memory.27,28
Screening Methods
Hebb William/ Rectangular maze
This maze is a reward-restricted behavioral model intended to assess rodents’ working memory. It is designed as an entirely sealed rectangular box having an entering chamber A and a rewarding chamber B attached at opposite ends. The rectangular box is segmented with wooden boards into blind passageways, leaving merely a twisting corridor C that leads from entering chamber A to rewarding chamber B.27 The learning assessment for all the control and treated rats was performed after the treatments. On the day one, all rats were acquainted with the rectangle maze for 10 minutes. From days two to five, the rats had four consecutive training trials every day in the maze, each lasting 8 to 10 minutes. In every trial, it was positioned in the entering chamber, and the timing device was activated when the rat exited the chamber. The time it took the rat to arrive in the rewarding chamber (TRC) was calculated as the day’s learning score. Lower assessment scores suggested sufficient learning, but rather higher scores suggested poor learning in rats. To warrant motivation to explore the rewarding area throughout the learning assessments, the rats were provided with water and food ad libitum for only one hour soon after the rectangle maze exposure of the day had been completed.
Elevated Plus Maze (EPM)
The EPM is utilized as an exteroceptive behavioral paradigm to assess memory and learning in rats. It comprises a 10×10 cm central platform connected with two 50×10 cm open- arms and two 50x40x10 cm closed arms. It is mounted 50 cm above the floor. The experiment was conducted in two stages. On the 14th day, acquisition testing was done. Each rat was positioned at the extremity of anyone open arm. They were positioned in such a way that they were facing opposite the central platform. The time required to enter either of the two closed arms has been documented as (TL) transfer latency. All four feet in a closed arm were considered as one entry. The cut-off time for every individual rat was 180 seconds. Rats that failed to enter any one of the closed arms before the specified period were eliminated from the research study. Retention tests were performed 24 hours following the initial trial, and the TL was recorded in the same manner as previously described. Shortened TL was perceived as an indicator of improvement in memory.29
Statistical Analysis
The results are expressed as mean ± standard error of the mean. To compare the variations in the parameters evaluated between the groups, a One-way ANOVA plus Dunnett’s Multiple Comparison Test was performed. A (p-value) probability value that was below 0.05 was inferred as statistically significant. SPSS version 25(‘IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp’) was utilized for statistical analysis
Results and Discussion
Hebb-William Maze
In the analysis of TRC in ‘Hebb-William Maze’ as shown in Table 2, ‘the time taken by the animal to reach the reward chamber’ was significantly increased in group 2 (negative control- scopolamine) on day 14 (160.00±7.60) and 15(149.67±9.21) when compared to the group 1(normal control) illustrating the amnesic effect of scopolamine. The TRC decreased significantly in group 3 (standard control-Piracetam) on days 14(82.50±3.43) and 15 (58.33±4.38) when compared to group 2 (negative control- scopolamine) demonstrating increase in memory and learning scores. The data revealed that the TRC on day 14 in group 4 treated with AETBFP 143 mg/kg was 118.50 ±4.39, group 5 treated with AETBFP 200 mg/kg was 112.83 ±2.37, and in group 6 treated with AETBFP 334 mg/kg was 109.33±4.77(p<0.05). On day 15 the TRC in the group 4 treated with AETBFP 143 mg/kg was 105.33±1.97, group 5 treated with AETBFP 200 mg/kg was 107.50±2.17, and group 6 treated with AETBFP 334 mg/kg was 99.50±3.31 (p<0.05). The above results depict a significant dose-dependent decline in the TRC when compared to the group 2 (negative control- scopolamine). Group 7 receiving AETBFP 334mg/kg and Piracetam decreased TRC considerably lower than other test groups on day 14 (103.67±3.39) and 15 (92.67±1.22) which was significant (p<0.05) when compared to the group 2 (negative control- scopolamine). There was a decrease in TRC in piracetam and AETBFP treated groups demonstrating their learning and memory enhancing effect in animal models. The TRC did not significantly differ within groups between days 14 and 15.
Table 2: Time to reach Reward Chamber in Hebb-William Maze on day 14 and 15
| Group | Drugs (dose) | Day 14 (TRC)
Acquisition Time in seconds |
Day 15
(TRC) Retention Time in seconds |
| 1 | Normal saline (1ml/kg b.w.) | 102.33±1.58 | 96±5.83 |
| 2 | Scopolamine (1mg/kg b.w.) | 160.00±7.60 | 149.67±9.21 |
| 3 | Piracetam (600mg/kg b.w.) | 82.50±3.43$ | 58.33±4.38$ |
| 4 | AETBFP (143mg/kg b.w.) plus Scopolamine (1mg/kg b.w.) | 118.50 ±4.39* | 105.33±1.97* |
| 5 | AETBFP (200mg/kg b.w.) plus Scopolamine (1mg/kg b.w.) | 112.83 ±2.37* | 107.50±2.17* |
| 6 | AETBFP (334mg/kg b.w.) plus Scopolamine (1mg/kg b.w.) | 109.33±4.77* | 99.50±3.31* |
| 7 | AETBFP (334mg/kg b.w.)) plus Piracetam (600 mg/kg b.w.) + Scopolamine (1mg/kg b.w.) | 103.67±3.39* | 92.67±1.22* |
AETBFP- ‘aqueous extract of Terminalia bellirica fruit pulp’, b.w- body weight
Values are expressed as mean± SEM, TRC-‘Time to reach reward chamber’.
$ p < 0.05 group 3 compared to group 1, * p < 0.05 test groups compared to group 2.
Elevated Plus Maze (EPM)
In the analysis of TL using an elevated plus maze as tabulated in table 3, ‘the time taken by the animal to enter any one of the closed arms’ significantly increased on day 14 (80.83±3.99) and 15 (72.17±3.92) in group 2 (negative control- scopolamine) when compared to normal control depicting the learning and memory impairment induced by scopolamine. The group 3 treated with piracetam showed a significant decrease in TL on day 14 (35.17±2.28) and 15(29.50±2.70) in comparison to group 2 (negative control- scopolamine) indicating an improvement in learning score and memory. The data revealed that the TL on day 14 in the group 4 treated with AETBFP 143 mg/kg was 62.00±2.62, group 5 treated with AETBFP 200 mg/kg was50.00±2.30(p<0.05), and in group 6 treated with AETBFP 334 mg/kg was 42.17±1.70(p<0.05). On day15 the TL in the group 4 treated with AETBFP 143 mg/kg was 54.50±2.69, group 5 administered with AETBFP 200 mg/kg was 41.67±.66(p<0.05), and group 6 treated with AETBFP 334 mg/kg was 35.50±1.38(p<0.05). The above results depict a dose dependent decrease in TL compared to group 2(negative control- scopolamine). The groups treated with AETBFP 200, 334 mg/kg showed a significant decrease in TL on both day 14 and 15. The group 7 receiving AETBFP 334 mg/kg and piracetam decreased TL on day 14(34.50±1.25) and day 15 ((2733±1.49) which was significant when compared to group 2(negative control- scopolamine). Though there was a decrease in TL in group treated with AETBFP and piracetam compared to piracetam alone, it was not significant. It was observed that there was no significant difference in the decrease in TL within groups on days 14 and 15.
Table 3: Effect on transfer latency using elevated plus maze on day 14 and 15
| Group | Drugs (dose) | Day 14
(TL) Acquisition Time in seconds |
Day 15
(TL) Retention Time in seconds |
| 1 | Normal saline (1ml/kg b.w.) | 39.33±2.12 | 34.17±1.01 |
| 2 | Scopolamine (1mg/kg b.w.) | 80.83±3.99 | 72.17±3.92 |
| 3 | Piracetam (600mg/kg b.w.) | 35.17±2.28$ | 29.50±2.70$ |
| 4 | AETBFP (143mg/kg b.w.) plus Scopolamine (1mg/kg b.w.) | 62.00±2.62 | 54.50±2.69 |
| 5 | AETBFP (200mg/kg b.w.) plus Scopolamine (1mg/kg b.w.) | 50.00±2.30* | 41.67±.66* |
| 6 | AETBFP (334mg/kg b.w.) plus Scopolamine (1mg/kg b.w.) | 42.17±1.70* | 35.50±1.38* |
| 7 | AETBFP (334mg/kg b.w.)) plus Piracetam (600 mg/kg b.w.) + Scopolamine (1mg/kg b.w.) | 34.50±1.25* | 27.33±1.49* |
AETBFP- ‘aqueous extract of Terminalia bellirica fruit pulp’, b.w- body weight
Values are expressed as mean± SEM, TL- ‘Transfer latency’.
$ p < 0.05 group 3 compared to group 1, * p < 0.05 test groups compared to group 2
Dementia is described as any medical condition in which a considerable decline in one’s prior level of cognitive ability impairs social, occupational and domestic functioning.30 In recent decades, dementia triggered by a variety of aetiologies has become more widespread among the elderly.31 Alzheimer’s disease constitutes the primary reason for dementia and has quickly grown into the most pricey, life-threatening and debilitating disease in this century.32 The brain gets exposed to oxidative damage. as its ‘reactive oxygen species’ (ROS) levels rise, and antioxidant defenses decline. Increased ROS levels may adversely affect cellular components or molecules, causing RNA, DNA, lipid, or protein oxidation. Oxidative damages are implicated in the molecular pathways that connect the build-up of two major aberrant proteins: extracellular “amyloid-beta “plaques and improperly phosphorylated “microtubule-associated protein tau” neurofibrillary tangles.33,34Terminalia bellirica fruit pulp contain phytoconstituents such as bellericanin, gallotannic acid, ellagic acid, termilignan, gallic acid, thannilignan, anolignanB, flavone, tannins, ethyl gallate, ellargic acid, galloyl, chebulaginic acid, glucose, phenyllemblin, sitisrerol, fructose, mannitol, and rhamnose.35 Secondary plant metabolites, phenol compounds and flavonoids exhibit antioxidant activities. The investigations proved that Terminalia bellerica fruit pulp possesses antioxidant activity by demonstrating significant reducing power, overall antioxidant capacity, hydroxyl radical scavenging and free radical scavenging.36 In light of the aforementioned, the following research was carried out.
As far as the researchers are aware, no prior pharmacological investigation into Terminalia bellerica fruit pulp’s efficacy against dementia in rodents has been documented. Scopolamine was utilized to induce Alzheimer’s-type dementia, while piracetam was employed as the standard control in both the Hebb’s William and the Elevated Plus Maze models. Scopolamine induces dementia of Alzheimer’s-type by enhancing inflammation, AChE levels and oxidative stress. These are found to be intimately connected to cognition and memory issues.37 Piracetam, a GABA derivative nootropic agent, has significant effectiveness in improving age-dependent cognitive impairment. It acts in the brain by modulating activating and inhibitory pathways, enhancing oxygen consumption, antioxidant actions, and increasing cholinergic receptors.38 Similarly, the test drug, Terminalia bellirica fruit pulp, may provide comparable antioxidant effects.
Research on aging, brain damage, and neurological illnesses have all made use of the ‘Hebb-Williams maze’ test, which is a popular and reliable technique for evaluating both rodents’ spatial learning and rodents’ memory. The maze is named after the designers, Richard Williams and Donald Hebb, who developed it as early as the 1940s.39,40
In ‘Hebbs William maze model’, (table 2) it was revealed that the TRC of group 4 (AETBFP 143 mg/kg) on day 14 (118.50 ±4.39) and day15 (105.33±1.97) and the TRC of group 5 (AETBFP 200 mg/kg) on day 14 (112.83 ±2.37) and day15 (107.50±2.17) were significantly reduced when compared to the group 2 (negative control- scopolamine) on the day 14 (160.00±7.60) and the day 15 (149.67±9.21), demonstrating adequate drug dosage resulting in an adequate drug concentration at the site of action. The TRC of group 6 (AETBFP 334mg/kg) on day 14 (109.33±4.77) and day 15 (99.50±3.31) and the TRC of group 7 (AETBFP 334mg/kg + Piracetam) on day 14 (103.67±3.39) and day 15 (92.67±1.22) significantly reduced when compared to the group 2 (negative control- scopolamine) on the day 14 (160.00±7.60) and the day 15 (149.67±9.21). A higher dosage has resulted in a higher concentration of the drug at the site of action bringing about elevated pharmacological response. A higher reduction in TRC is noted in group 7 (AETBFP 334mg/kg + Piracetam) compared to group 6 (AETBFP 334mg/kg) which is due to the addition of standard drug Piracetam to the test drug. When TRC of day 14 (acquisition parameter) and day 15 (memory retention parameter) were compared in figure 1, it is shown that there was a minor drop of TRC on day 15 in all the groups, this can be attributed to retrieval of the stored memory.
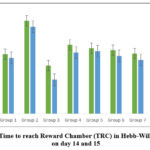 |
Figure 1: Time to reach Reward Chamber (TRC) in Hebb-William Maze on day 14 and 15. |
The experimental model, elevated plus maze model is a widely accepted methodology for training, memory, and learning processes in rodents. It is made up of open and closed arms arranged perpendicularly, which are crossed in the centre by a platform. Rodents are free to navigate the maze amid open and closed arms. The amount of time that takes for an animal to locomote from one open arm to one closed arm is the TL. The reduction in TL serves as an indicator of memory. 41
In the elevated plus maze model, (table 3) it was revealed that group 4 did not show any significant decrease in TL on day 14 and 15 which may be because of the inadequate drug concentration at the site of action. TL of group 5 (AETBFP 200 mg/kg) on day 14 (50.00±2.30) and day15 (41.67±.66) and TL of group 6 (AETBFP 334mg/kg) on day 14 (42.17±1.70) and day 15 (35.50±1.38) were significantly reduced when compared to the group 2 (negative control- scopolamine) on the day 14 (80.83±3.99) and the day 15 (72.17±3.92), demonstrating adequate drug dosage resulting in an adequate drug concentration at the site of action. The TL of group 7 (AETBFP 334mg/kg + Piracetam) on the day 14 (34.50±1.25) and the day 15 (27.33±1.49) significantly reduced when compared to the group 2 (negative control- scopolamine) on the day 14 (80.83±3.99) and the day 15 (72.17±3.92). A higher reduction in TL is noted in group 7 (AETBFP 334mg/kg + Piracetam) which can be ascribed to a higher concentration of the drug at the site of action and the addition of standard drug Piracetam to the test drug bringing about the elevated pharmacological response. When TL of the day 14 (acquisition parameter) and day 15 (memory retention parameter) were compared in figure 2, it is shown that there was a minor drop of TL on day 15 in all the groups, this can be linked to retrieval of the consolidated memory.
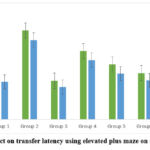 |
Figure 2: Effect on transfer latency using elevated plus maze on day 14 and 15 |
Conclusion
In essence, ‘the aqueous extract of Terminalia bellirica fruit pulp’ revealed potential anti-dementia efficacy in rat brain tissue. A significant cognitive enhancing effect was reported at extraction dosages of 200, and 334mg/kg. By the occurrence of organic materials such as flavonoids, phenol groups and tannins, the plant material’s antioxidant potential could be attributed to the neuroprotective action. In this viewpoint, natural compounds are valuable leads in the discovery of innovative drugs, having a potential significance in medical treatments. Further studies focusing on the determination of the chemical structure of molecules, pharmacokinetics, and pharmacodynamics that are liable for the cognition-enhancing activity can be explored.
Acknowledgement
We are grateful to Kasturba Medical College, Mangalore, Manipal Academy of Higher Education, for providing us with amenities for this research project.
Funding source
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest
The author(s) do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
The protocol received Institutional Animal Ethics Committee (IAEC) approval for a three-month research period involving forty-two ‘Wistar albino rats’ (IAEC approval number: KMC.MNG/IAEC/08/2023). The CCSEA registration number assigned to the IAEC is 213/PO/Re/S/2000/CPCSEA07/09/2022 (Valid up to 06/09/2027). The rodents were reared in the Pharmacology department’s animal house, KMC Mangalore, Karnataka, India. The research study was conducted in the Experimental laboratory, Pharmacology department, Kasturba Medical College, Mangalore, India.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required
Clinical Trial Registration
This research does not involve any clinical trials
Authors’ Contribution
Poovizhi Bharathi R- Conceptualization, Formal analysis, Funding acquisition Project administration; Software; Supervision; Validation; Visualization; Roles/Writing – original draft; and Writing – review & editing, Shamitha Rai- Data curation; Funding acquisition; Investigation; Methodology, Rashmi R Rao- Conceptualization, Formal analysis, Supervision, Validation; Visualization, Roles/Writing – original draft; and Writing – review & editing, Trishna Sudarshan- Data curation; Funding acquisition; Investigation; Methodology , Ashitha Leslie Mariam- Investigation; Methodology. Each author mentioned has significantly and directly contributed intellectually to the project and has given their approval for its publication.
References
- Assal F. History of Dementia. Front Neurol Neurosci. 2019; 44:118-126.
CrossRef - Duong S, Patel T, Chang F. Dementia: What pharmacists need to know. Can Pharm J (Ott). 2017;150(2):118-129.
CrossRef - Wendy W. Statement on donepezil and anti-dementia medications for the 24th WHO Essential Medicines List meeting. Alzheimer’s Disease International. https://www.alzint.org/resource/statement-on-donepezil-and-anti-dementia-medications-for-the-24th-who-essential-medicines-list-meeting/. Published April 24, 2023. Accessed September 13, 2024.
- World Health Organisation. Dementia. https://www.who.int/news-room/fact-sheets/detail/dementia.Published March 15, 2023. Accessed September 13, 2024.
- Alzheimer’s Association. 2012 Alzheimer’s disease facts and figures. Alzheimers Dement. 2012;8(2):131-168.
CrossRef - Błaszczyk JW. Pathogenesis of Dementia. Int J Mol Sci. 2022;24(1):543.
CrossRef - Monteiro AR, Barbosa DJ, Remiao F, Silva R. Alzheimer’s disease: Insights and new prospects in disease pathophysiology, biomarkers and disease-modifying drugs. Biochem Pharmacol. 2023; 211:115522.
CrossRef - Kumar A, Gupta V, Sharma S. Donepezil. In: Stat Pearls . Treasure Island (FL): StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK513257/.Updated Aug 17, 2023. Accessed September 13, 2024.
- McShane R, Westby MJ, Roberts E, Minakaran N, Schneider L, Farrimond LE, Maayan N, Ware J, Debarros J. Memantine for dementia. Cochrane Database Syst Rev. 2019;3(3):CD003154.
CrossRef - Guo M, Qin S, Wang S, Sun M, Yang H, Wang X, Fan P, Jin Z. Herbal Medicine Nanocrystals: A Potential Novel Therapeutic Strategy. 2023;28(17):6370.
CrossRef - Shaito A, Thuan DTB, Phu HT, Nguyen THD, Hasan H, Halabi S, Abdelhady S, Nasrallah GK, Eid AH, Pintus G. Herbal Medicine for Cardiovascular Diseases: Efficacy, Mechanisms, and Safety. Front Pharmacol. 2020;11:422.
CrossRef - Tesfaye R, Degu A, Abebe B, Ayalew H. Evaluation of Analgesic and Anti-inflammatory Potential of 80% Methanol Leaf Extract of Otostegia integrifolia Benth (Lamiaceae). J Inflamm Res. 2020; 13:1175-1183.
CrossRef - Kong Q, Shang Z, Liu Y, Fakhar-E-Alam Kulyar M, Suo-Lang S, Xu Y, Tan Z, Li J, Liu S. Preventive effect of Terminalia bellirica (Gaertn.) Roxb. extract on mice infected with Salmonella Typhimurium. Front Cell Infect Microbiol.2023;12:1054205.
CrossRef - Jayesh K, Helen LR, Vysakh A, Binil E, Latha MS. Protective Role of Terminalia bellirica(Gaertn.) Roxb Fruits Against CCl4 Induced Oxidative Stress and Liver Injury in Rodent Model. Indian J Clin Biochem. 2019;34(2):155-163.
CrossRef - Umesh Kanna S, Parthiban KT, Senthilraja K, Venkatesan S, Udhaya Nandhini D, Mohan Kumar S, Dhasarathan M, Kumaresan P, Sai MJ, Raveendran M, Geethalakshmi V. Genetic Diversity and Structure of Terminalia bellerica(Gaertn. Roxb.) Population in India as Revealed by Genetic Analysis. Plants (Basel). 2024;13(4):470.
CrossRef - Roxburgh W, Banks J, Bulmer W, Mackenzie D, Nicol G. Plants of the Coast of Coromandel: Selected from Drawings and Descriptions Presented to the Hon. Court of Directors of the East India. 2. London:W. Bulmer and Co. for G. Nicol, Bookseller; 1798.
- Deb A, Barua S, Das B. Pharmacological activities of Baheda (Terminalia bellerica): A review. J Pharmacogn Phytochem, 2016;5(1):194-197.
- Gupta A, Kumar R, Bhattacharyya P, Bishayee A, Pandey AK. Terminalia bellirica (Gaertn.) roxb. (Bahera) in health and disease: A systematic and comprehensive review. 2020;77:153278.
CrossRef - Biswajit D, Suvakanta D, Chandra CR. Pharmaceutical properties of Terminalia bellerica (Bahera)-an overview. RJPT. 2014; 7(5): 592–597.
- Akhtar J, Bashir F. Terminalia bellerica (Bahera): Antidiabetic and Other Potential Benefits. In: Antidiabetic Medicinal Plants and Herbal Treatments. Boca Raton, Florida: CRC Press; 2023.
CrossRef - Peterson CT, Denniston K, Chopra D. Therapeutic Uses of Triphala in Ayurvedic Medicine. J Altern Complement Med. 2017;23(8):607-614.
CrossRef - Kim MS, Lee DY, Lee J, Kim HW, Sung SH, Han JS, Jeon WK. Terminalia chebula extract prevents scopolamine-induced amnesia via cholinergic modulation and anti-oxidative effects in mice. BMC Complement Altern Med. 2018;18(1):136.
CrossRef - Justin Thenmozhi A, Dhivyabharathi M, William Raja TR, Manivasagam T, Essa MM. Tannoid principles of Emblica officinalis renovate cognitive deficits and attenuate amyloid pathologies against aluminum chloride induced rat model of Alzheimer’s disease. Nutr Neurosci. 2016;19(6):269-278.
CrossRef - Husain I, Akhtar M, Madaan T, Vohora D, Abdin MZ, Islamuddin M, Najmi AK. Tannins Enriched Fraction of Emblica officinalis Fruits Alleviates High-Salt and Cholesterol Diet-Induced Cognitive Impairment in Rats via Nrf2-ARE Pathway. Front Pharmacol. 2018;9:23.
CrossRef - Verma DK, Joshi N, Raju KS, Wahajuddin M, Singh RK, Singh S. Metabolic enhancer piracetam attenuates rotenone induced oxidative stress: a study in different rat brain regions. Acta Neurobiol Exp (Wars). 2015;75(4):399-411.
CrossRef - Kumar B, Divakar K, Tiwari P, Salhan M, Goli D. Evaluation of anti-diarrhoeal effect of aqueous and ethanolic extracts of fruit pulp of Terminalia belerica in rats. IJDDR. 2010;2(4):769-779.
- Gupta A, Kumar R, Pandey AK. Antioxidant and antidiabetic activities of Terminalia bellirica fruit in alloxan induced diabetic rats. S African J Bot. 2020;130:308–315.
CrossRef - Vasundhara S, Hafsa A, Rajiv G. Memory Enhancing Effects Of Ficus Carica Leaves In Hexane Extract On Interoceptive Behavioral Models. Asian J Pharm Clin Res.2013;6(3):109-113.
- Vijayalakshmi, Adiga S, Bhat P, Chaturvedi A, Bairy KL, Kamath S. Evaluation of the effect of Ferula asafoetida Linn. gum extract on learning and memory in Wistar rats. Indian J Pharmacol. 2012;44(1):82-87.
CrossRef - Gale SA, Acar D, Daffner KR. Dementia. Am J Med. 2018;131(10):1161-1169.
CrossRef - Raz L, Knoefel J, Bhaskar K. The neuropathology and cerebrovascular mechanisms of dementia. J Cereb Blood Flow Metab. 2016;36(1):172-186.
CrossRef - Cheltens P, De Strooper B, Kivipelto M, Holstege H, Chetelat G, Teunissen CE, Cummings J, van der Flier WM. Alzheimer’s disease. Lancet. 2021;397(10284):1577-1590.
CrossRef - Buccellato FR, D’Anca M, Fenoglio C, Scarpini E, Galimberti D. Role of Oxidative Damage in Alzheimer’s Disease and Neurodegeneration: From Pathogenic Mechanisms to Biomarker Discovery. Antioxidants (Basel). 2021;10(9):1353.
CrossRef - Schneider JA. Neuropathology of Dementia Disorders. Continuum (Minneap Minn). 2022;28(3):834-851.
CrossRef - Kumar N, Khurana SMP. Phytochemistry and medicinal potential of the Terminalia bellirica Roxb. (Bahera). Indian J Nat Prod Resour. 2018;9(2):97-107.
- Gupta A, Kumar R, Pandey AK. Antioxidant and antidiabetic activities of Terminalia bellirica fruit in alloxan induced diabetic rats. S African J Bot. 2020;130:308–315.
CrossRef - Foudah AI, Devi S, Alam A, Salkini MA, Ross SA. Anticholinergic effect of resveratrol with vitamin E on scopolamine-induced Alzheimer’s disease in rats: Mechanistic approach to prevent inflammation. Front Pharmacol. 2023;14:1115721.
CrossRef - Elbeltagy M, Khraisat B, AlZoubi L, Hmoud L, AlJeady A, Yousef M, Salman A. The neuroprotective effects of Piracetam on cisplatin-induced cognitive decline. Int J Neurosci. 2023;28:1-8.
CrossRef - Kishore GK, Venkateshu K, Sridevi N. Effect of 1800-2100 MHz Electromagnetic Radiation on Learning-Memory and Hippocampal Morphology in Swiss Albino Mice. J Clin of Diagn Res.2019; 13(2):AC14-AC17.
CrossRef - Hebb DO, Williams K. A method of rating animal intelligence. J Gen Psychol. 1946;34:59-65.
CrossRef - Parameshwari K, Shashikumara, Neeta CS, Prathima C. Investigation on learning and memory enhancing activity of Saraca asoca flower (Roxb.) Wilde in experimental mice. Natl J Physiol Pharm Pharmacol. 2018;8(9):1250-1255.
CrossRef







