Manuscript accepted on :05-12-2024
Published online on: 30-12-2024
Plagiarism Check: Yes
Reviewed by: Dr. Armerinayanti Pranata
Second Review by: Dr. Joyeeta Bhattacharya
Final Approval by: Dr. Eman Refaat Youness
Putu Anda Tusta Adiputra1* , I Wayan Sudarsa1
, I Wayan Sudarsa1 , Gede Budhi Setiawan1
, Gede Budhi Setiawan1 , Ida Bagus Made Suryawisesa1
, Ida Bagus Made Suryawisesa1 and Kadek Yudi Fajar Mahendra2
and Kadek Yudi Fajar Mahendra2
1Division of Surgical Oncology, Department of Surgery, Faculty of Medicine, Udayana University, Bali, Indonesia
2Division of Surgical Oncology, Department of Surgery, Bali Mandara Hospital, Bali, Indonesia
Corresponding Author E-mail: andatusta@unud.ac.id
DOI : https://dx.doi.org/10.13005/bpj/3035
Abstract
Mammaglobin (hMAG) is a secretory protein (secretoglobin) essential for tumor growth. The molecular mechanisms of hMGB-A-regulated growth include the expression and activation of various Mitogen-Activated Protein Kinase (MAPK) pathways. Elevated hMAG signifies metastatic breast cancer and primary breast cancer compared to non-malignant breast tissue. This study aimed to prove the relationship between mammaglobin A expression and metastasis at Prof. I.G.N.G. Ngoerah Hospital Bali. This is an observational study with a case-control design, conducted in the Oncology Department of Prof. I.G.N.G. Ngoerah Hospital Bali. Secondary data were collected from medical records which included the results of clinical data, histopathology, and immunohistochemistry. Data analysis was carried out with the help of SPSS version 25, which included the stages of descriptive analysis, bivariate tests with Chi-Square, Mann Whitney, and independent t-test. A total of 48 subjects were divided into 2 groups, 24 subjects in the metastasis group and 24 subjects in the non-metastasis group. Age, menstrual status, parity, histopathological grading, and breast cancer subtypes showed no significant differences between the two groups. The metastatic group had a larger tumor size with more lymph node metastasis than the non-metastatic group (p<0.001). The most common metastatic organ was in the lungs. The difference in the presence of Mammaglobin A expression in metastases had an RR of 22.0 (95%CI = 4.1-117.8; p<0.001) with the results of the percentage of strong expression with the metastatic group having a mean of 55.0% ± 31.8% and not metastatic 7.5% ± 11.9% (p<0.001). The results of weak expression found no significant difference in the two groups with a value of p=0.376. In conclusion, strong mammaglobin A expression increase the risk of metastasis in breast cancer by 22 times.
Keywords
Breast Cancer; Mammaglobin A; Metastasis; Risk Factor; Tumor Tissue
Download this article as:| Copy the following to cite this article: Adiputra P. A. T, Sudarsa I. W. Y, Setiawan G. B, Suryawisesa I. B. M, Mahendra K. Y. F. Strong Mammaglobin-A Expression in Tumor Tissue as a Risk Factor for Metastasis in Balinese Breast Cancer Patients. Biomed Pharmacol J 2024;17(4). |
| Copy the following to cite this URL: Adiputra P. A. T, Sudarsa I. W. Y, Setiawan G. B, Suryawisesa I. B. M, Mahendra K. Y. F. Strong Mammaglobin-A Expression in Tumor Tissue as a Risk Factor for Metastasis in Balinese Breast Cancer Patients. Biomed Pharmacol J 2024;17(4). Available from: https://bit.ly/4gRo4k5 |
Introduction
Based on Globocan 2022 data, breast cancer occupied 11.6% of it, or around 2,296,840 people affected. The incidence of breast cancer in America is around 92/100,000 women with an 18% mortality rate.1 Based on the Pathology Based Registration in Indonesia, breast cancer ranks first with a prevalence of 18.6%. It is estimated that the incidence rate of breast cancer in Indonesia is 12/100,000 women.2
Metastasis in breast cancer is the migration of breast malignant cells to other tissues of the body. Metastasis indicates the formation of a secondary implant located far from the primary tumor. Breast cancer metastasis can spread through lymphatic system to the lymph nodes, and hematogenously to other organs. The most often affected organs by distant metastases from breast cancer include the brain (less than 5%), pleura (5-15%), lung (5-15%), bone (40-75%), and liver (3-10%). Both hematogenous and lymphogenous metastasis pose a serious threat to breast cancer patients.3
Metastasis detection is essential in breast cancer patients because it significantly affect the long-term survival of the patients. The earliest detection of metastasis in breast cancer patients is better, since many of the patients, especially in Indonesia is diagnosed in late stage. There are many biomarker available for breast cancer such as carcinoembryonic antigen (CEA), cancer antigen (CA) 15-3, CA 27-29. But these biomarkers still has several limitation, one of them is lack of sensitivity. 4 Mammaglobin (hMAG) A, also known as Mammaglobin (MGB) 1 or Uteroglobin (UGB) 2 or Secretoglobin (SCGB) 2A2, is only overexpressed in breast cancer.5 Mammaglobin A, which belongs to the secretory protein family, is overexpressed in 40–80% of instances of breast cancer, both primary and metastatic. Previous study found that Balinese race with metastatic breast cancer has higher mRNA expression. This showed Mammaglobin A is employed as a diagnostic biomarker in individuals with breast cancer because of its distinct and universal expression features.4 Mammaglobin A expression profile is specific to the breast organ, so Mammaglobin A is considered a prominent biomarker for breast cancer cell detection. Currently, Mammaglobin A can be used as a clinical biomarker to detect metastatic breast cancer larger than 0.2 mm2.6–8 Previous study evaluated Mammaglobin A is only focus in breast cancer in general, and lack information regarding the effect of Mammaglobin A expression in tumor tissue with the risk of metastasis. Therefore, the purpose of this study was to assess the association between mammaglobin A expression and metastasis in Balinese patients with breast cancer.
Materials and Methods
This study is a case-control study, started from January to December 2023 in the medical record room, anatomic pathology, and data from the Oncology department of Prof. I.G.N.G. Ngoerah Hospital Bali. All Balinese patients with breast cancer who underwent treatment at Prof. I.G.N.G. Ngoerah Hospital Bali with complete medical records were included in this study. Meanwhile, the exclusion criteria in this study included breast cancer patients who had performed immunohistochemical examinations outside Prof. I.G.N.G. Ngoerah Hospital Bali and male breast cancer patients.
This study evaluated the clinicopathological data which includes age, menstrual status, tumor size, parity, distant metastasis, lymph node metastasis, histopathological grading, subtypes, collected from the medical record, and Mammaglobin A expression which was carried out by immunohistochemistry (IHC) of the formalin-fixed paraffin-embedded specimen using monoclonal antibody anti-Mammaglobin A. Two pathologists then assessed the IHC data using a 0–3 scoring system depending on the expression following staining (Table 1). A score of 0-1 is categorized as a weak expression, while a score 2 is categorized as a strong expression.
Table 1: Mammaglobin A category based on IHC results
| IHC results | Scoring |
| Strong staining | 3 |
| Moderate staining | 2 |
| Weak staining | 1 |
| No staining | 0 |
The data were analyzed using the SPSS software. The stages of data analysis were as follows: univariate analysis, bivariate analysis using Chi-square test to obtained Relative risk (RR) and independent t-test or Mann-Whitney for numerical data. The results are considered significant if p-value <0.05.
Results
A total of 48 research subjects were recruited for this study and split into two groups: 24 subjects were assigned to the metastasis group and another 24 patients to the non-metastasis group. Table 2 displays the data characteristics results.
Table 2: Data Characteristics
| Variables | Group | P-value | |
| Metastasis (n=24) | No metastasis (n=24) | ||
| Age, mean±SD | 46.00±13.03 | 48.00±7.07 | 0.905a |
| Menstrual Status, n (%) | |||
| Premenopause | 9 (37.5%) | 6 (25.0%) | 0.350b |
| Postmenopause | 15 (62.5%) | 18 (75.0%) | |
| Parity, mean±SD | 2.00±1.50 | 2.5±0.70 | 0.486 a |
| Tumor size, n (%) | |||
| T1 | 0 (0.0%) | 1 (4.2%) | <0.001b |
| T2 | 0 (0.0%) | 22 (91.7%) | |
| T3 | 4 (16.7%) | 1 (4.2%) | |
| T4 | 1 (4.2%) | 0 (0.0%) | |
| T4a | 1 (4.2%) | 0 (0.0%) | |
| T4b | 13 (54.2%) | 0 (0.0%) | |
| T4c | 4 (16.7%) | 0 (0.0%) | |
| T4d | 1 (4.2%) | 0 (0.0%) | |
| Lymph node metastasis, n (%) | |||
| 0 | 0 (0.0%) | 11 (45.8%) | <0.001*b |
| 1 | 14 (58.3%) | 12 (50.0%) | |
| 2 | 6 (25.0%) | 1 (4.2%) | |
| 3 | 4 (16.7%) | 0 (0.0%) | |
| Histopathology grading, n (%) | |||
| 1 | 3 (12.5%) | 2 (8.3%) | <0.406b |
| 2 | 8 (33.3%) | 13(54.2%) | |
| 3 | 13 (54.2%) | 9 (37.5%) | |
| Number of organ metastases, n (%) | |||
| 1 | 12 (50.0%) | ||
| 2 | 10 (41.7%) | ||
| 3 | 2 (8.3%) | ||
| Subtypes, n (%) | |||
| Luminal A | 3 (12.5%) | 6 (25.0%) | 0.071b |
| Luminal B | 3 (12.5%) | 4 (16.7%) | |
| Luminal-HER 2 | 3 (12.5%) | 5 (20.8%) | |
| HER 2 | 6 (25.0%) | 5 (20.8%) | |
| TNBC | 9 (37.5%) | 4 (16.7%) | |
Notes: aindependent t-test,b Chi-Square,*Significant
The highest number of organ metastases obtained was 1 location, the details of which are presented in Table 3 with the most results being the lung area with the results of 5 (20.8%) subjects.
Table 3: Location of distant metastasis
| Location of distant metastasis | n (%) |
| Lung | 5 (20.8%) |
| Lung, liver | 4 (16.7%) |
| Hepar | 3 (12.5%) |
| Lungs, bones | 3 (12.5%) |
| Bones | 2 (8.3%) |
| Brain | 1 (4.2%) |
| Bone, spine | 1 (4.2%) |
| Lung, contralateral of breast | 1 (4.2%) |
| Lung, liver, brain | 1 (4.2%) |
| Lung, bone, contralateral of breast | 1 (4.2%) |
| Hepar, bone | 1 (4.2%) |
| Lung, liver, brain | 1 (4.2%) |
The results of IHC on Mammaglobin A can be seen in Figure 1. This study found 18 (37.5%) patients categorized as having strong expression of Mammaglobin A, while 30 (62.5%) others had weak expression of Mammaglobin A.
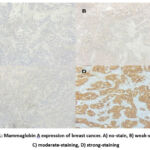 |
Figure 1: Mammaglobin A expression of breast cancer. A) no-stain, B) weak-staining, C) moderate-staining, D) strong-staining.Click here to View Figure |
Table 3 presents an analysis of the association between metastasis and mammaglobin A expression. It showed that 66.7% of samples with breast cancer metastases had strong Mammaglobin A expression. Meanwhile, the no metastasis showed that 91.7% of the patients had a weak expression of Mammaglobin A. It also showed that patients with strong expression of Mammaglobin A have a 22.00 times higher risk of developing metastasis compared to patients with negative or low expression of Mammaglobin A. This showed that patients with breast cancer who have spread compared to those who have not had any metastases have significantly different expression levels of mammaglobin A (p<0.001; 95%CI: 4.10 – 117.80) (Table 4).
Table 4: Association between metastasis and mammaglobin A expression
| Variables | Group | OR | P-value | 95%CI | |
| Metastasis | No metastasis | ||||
| Mammaglobin A | |||||
| Strong expression (≥2) | 16 (66.7%) | 2 (8.3%) | 22.00 | <0.001a | 4.10 – 117.80 |
| Weak expression (0-1) | 8 (33.3%) | 22 (91.7%) | |||
Description: a Chi-Square
Based on the 2×2 bivariate chi-square analysis, these results indicate that the sensitivity rate of Mammaglobin A as a metastasis marker is quite good (66.7%), and has a high positive predictive value (PPV) (88.9%). This indicates that when the patient’s Mammaglobin A result is positive, it has a positive prediction rate of 88.9% for the occurrence of metastasis (Table 5).
Table 5: Diagnostic value of Mammaglobin A in metastatic breast cancer
| Marker | Sensitivity | Specificity | Positive Predictive Value (PPV) | Negative Predictive Value (NPV) |
| Mammaglobin A | 66.7 | 91.7 | 88.9 | 73.3 |
Discussion
Age, menstrual state, parity, histopathological grade, and breast cancer subtype did not significantly differ from the incidence of metastasis. But eventually, there were significant differences in tumor size and the presence of lymph node metastasis between the two groups. The most common location for distant metastasis in this study is the lung. This is consistent with a prior study that discovered lung metastases were identified in 60–70% of breast cancer patients who ultimately passed away.9 Breast cancer has a tendency to metastasize certain organs, most often the bones and lungs, liver, and brain.10 Most mortality from breast cancer are caused by metastasis, or the tumor cells spreading throughout the body. The survival rate for individuals with lung metastasis is still extremely low, despite advancements in the numerous medicines available for the condition, including as targeted therapy, chemotherapy, and radiotherapy.11
Breast cancer is a form of malignant tumor that is commonly seen in clinical settings. It varies widely in terms of molecular subtypes, therapeutic options, and the degree of disease variety. The majority of patients’ safety, quality of life, and physical and mental health are all impacted by this diversity, which also has an impact on the prognosis of the condition. Consequently, early detection of breast cancer depends on pathological diagnosis at the molecular level, which calls for special attention in the development of more precise detection techniques and the identification of useful indicators for diagnostic and prognostic reasons.12
One well-known particular marker for breast cancer is mammaglobin expression, which is positively correlated with tumor stage, histological grade, lymph node metastasis, and endocrine status. In this investigation, we discovered a strong correlation between breast cancer patients’ metastases and strong expression of mammaglobin A. This discovery is consistent with earlier research that found mammaglobin overexpression to be a possible marker of metastasis in breast cancer patients and linked to metastasis to lymph nodes.12
These results are consistent with a prior study which discovered that a poor prognosis was related to positive expression of mammaglobin A (P<0.001; 95%CI 1.48-2.91).13 Another case-control study also found that overexpression of Mammaglobin A mRNA was a risk factor for breast cancer by 9 times (p = 0.002; 95% CI = 2.15 – 37.66) compared to low Mammaglobin A mRNA expression. In addition, Mammaglobin A mRNA overexpression increased the risk of breast cancer metastases by 7.36-fold (p = 0.013; 95% CI = 1.34 – 40.55).6
Mammaglobin A shows an excellent ability to detect breast cancer. This can be seen in the Positive Predictive Value (PPV) value of Mammaglobin A against breast cancer metastasis of 88.9%. This shows that, a total of 88.9% of patients with mammaglobin A expression will experience metastasis, in this study mostly in the lung, liver, and or bone organs. This is in line with the previous study which found mammaglobin A has a specificity of 100% and sensitivity of 81.5%.12 Furthermore, Mammaglobin A continues to have the highest specificity (100%) and a strong PPV value (74%) when compared to CEA and CA15-3.14 However, this study still has several limitations such as this is only a case-control study with a lack of time to evaluate the disease progression.
Conclusion
Balinese breast cancer patients with strong Mammaglobin A expression in tumor tissue has higher risk of metastasis.
Acknowledgment
The authors would like to thank Pathologist at Prof. Dr. I.G.N.G Ngoerah Denpasar Hospital Bali for providing the expertise and experience in tumor tissue examination
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The author(s) do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research has received a research permit from the research ethics committee of Udayana University / Prof. I.G.N.G. Ngoerah Hospital Bali with number 388 / UN14.2.2.VII.14 / LT / 2023.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials.
Author’s Contribution
Putu Anda Tusta Adiputra: Conceptualization, Analysis, Writing – Review & Editing
I Wayan Sudarsa: Methodology, Analysis, Writing – Review & Editing
Gede Budhi Setiawan: Data Collection, Analysis, Writing – Review & Editing
Ida Bagus Made Suryawisesa: Conceptualization, Analysis, Writing – Review & Editing
Kadek Yudi Fajar Mahendra: Data Collection, Writing – Original Draft
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249.
CrossRef - Samosir BP, Angka RN, Christina S, Endarjo S, Pandansari P. Gambaran Pasien dengan Benjolan Payudara yang Diperiksa Biopsi Aspirasi Jarum Halus di Yayasan Kanker Indonesia Tahun 2014-2018. Jurnal Kedokteran Meditek. 2021;27(1):9–15.
CrossRef - Yoon HY, Kim HN, Lee SH, Kim SJ, Chang Y, Ryu S, Shin H, Kim HL & Lee JH. Association between Neutrophil-to-Lymphocyte Ratio and Gut Microbiota in a Large Population: a Retrospective Cross-Sectional Study. Sci Rep. 2018;8(1):16031.
CrossRef - Al-Joudi FS, Kaid FA, Ishak I, Mohamed N, Osman K, Alias IZ. Expression of human mammaglobin and clinicopathologic correlations in breast cancer: the findings in Malaysia. Indian J Pathol Microbiol. 2011;54(2):284-289.
CrossRef - Kong X, Wang Q, Li J, Li M, Deng F, Li C. Mammaglobin, GATA-binding protein 3 (GATA3), and epithelial growth factor receptor (EGFR) expression in different breast cancer subtypes and their clinical significance. Eur J Histochem. 2022;66(2):3315. Published 2022 Apr 7.
CrossRef - Suryawisesa IBM, Manuaba IBTW. Overexpression of Mammaglobin-A in Primary Breast Tissue Tumor and High Concentration of mRNA Mammaglobin-A in Peripheral Blood as Risk Factors for Metastatic Breast Cancer. Asian Pacific Journal of Cancer Biology. 2024;9(2):129–35.
CrossRef - Marchetti A, Buttitta F, Bertacca G, Zavaglia K, Bevilacqua G, Angelucci D. mRNA markers of breast cancer nodal metastases: comparison between mammaglobin and carcinoembryonic antigen in 248 patients. J Pathol. 2001;195(2):186–90.
CrossRef - Guan X feng, Hamedani MK, Adeyinka A, Walker C, Kemp A, Murphy LC. Relationship Between Mammaglobin Expression and Estrogen Receptor Status in Breast Tumors. Endocrine. 2003;21(3):245–50.
CrossRef - Jin L, Han B, Siegel E, Cui Y, Giuliano A, Cui X. Breast cancer lung metastasis: Molecular biology and therapeutic implications. Cancer Biol Ther. 2018;19(10):858-868.
CrossRef - Gerratana L, Davis AA, Polano M. Understanding the organ tropism of metastatic breast cancer through the combination of liquid biopsy tools. Eur J Cancer. 2021;143:147-157.
CrossRef - Yousefi H, Vatanmakanian M, Mahdiannasser M, Mashouri L, Alahari N V, Monjezi MR. Understanding the role of integrins in breast cancer invasion, metastasis, angiogenesis, and drug resistance. Oncogene. 2021;40(6):1043–63.
CrossRef - Gorbokon N, Timm P, Dum D. Mammaglobin-A Expression Is Highly Specific for Tumors Derived from the Breast, the Female Genital Tract, and the Salivary Gland. Diagnostics (Basel). 2023;13(6):1202.
CrossRef - Hu Y, Liu P, Wu D, Jiang Y. Prognostic role of plasma mammaglobin A expression in breast carcinoma patients: a meta-analysis. Onco Targets Ther. 2018;11:3245-3255.
CrossRef - Burstein HJ, Lacchetti C, Anderson H,. Adjuvant Endocrine Therapy for Women With Hormone Receptor-Positive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update on Ovarian Suppression. J Clin Oncol. 2016;34(14):1689-1701.
CrossRef







