Ahmed Subeh Alshrari1 , Shuaibu Abdullahi Hudu2,3*
, Shuaibu Abdullahi Hudu2,3* , Abdulgafar Olayiwola Jimoh4
, Abdulgafar Olayiwola Jimoh4 and Bahaa Mohammed Badr2,5
and Bahaa Mohammed Badr2,5
1Medical Laboratory Technology Department, Faculty of Applied Medical Science, Northern Border University, Arar, Saudi Arabia
2Department of Basic and Clinical Medical Sciences, Faculty of Dentistry, Zarqa University, Zarqa, Jordans
3Department of Medical Microbiology and Parasitology, Faculty of Basic Clinical Sciences, College of Health Sciences, Usmanu Danfodiyo University, Sokoto, Sokoto State, Nigeria.
4Department of Pharmacology and Therapeutics, Faculty of Basic Clinical Sciences, College of Health Sciences, Usmanu Danfodiyo University, Sokoto, Sokoto State, Nigeria.
5Department of Medical Microbiology and Immunology, Faculty of Medicine, Al-Azhar University, Assiut, Egypt.
Corresponding Author E-mail: shudu@zu.edu.jo
DOI : https://dx.doi.org/10.13005/bpj/3019
Abstract
Genital herpes caused by herpes simplex virus infection type 2 (HSV-2) is one of the most common STDs that causes a substantial illness burden globally, particularly in sub-Saharan Africa. This study was aimed at determining the seroprevalence rate of HSV-2 in patients who presented at a Sokoto speciality hospital with fever. We collected 184 blood samples from consenting patients and used an ELISA to analyze them for HSV-2 antibodies. Sociodemographic and clinical data were also obtained from the patients via questionnaires before sample collection. The study found HSV-2 seroprevalence to be 54.3% (100 out of 184 patients). All patients aged 43-47 years (100%, 6 out of 6) tested positive for HSV-2 antibodies, while only two patients over the age of 53 tested positive (p = 0.729). The prevalence was higher among males (67.7%, 42 out of 62) compared to females (47.5%, 55 out of 122) (p = 0.066), and higher in single individuals (63.9%, 46 out of 72) than in married individuals (48.2%, 54 out of 112) (p = 0.141). Regarding occupational status, the highest prevalence was observed among employed patients (68.2%, 30 out of 44), followed by self-employed individuals (55.1%, 54 out of 98). More than half of the patients were infected with HSV-2, indicating a need for further studies to identify the risk factors associated with acquiring the virus. Increasing awareness about transmission routes and the potential consequences of HSV-2 infection is also crucial.
Keywords
Herpes; HSV-2; Sero-prevalence; Sexually transmitted infections; Sub-Saharan Africa
Download this article as:| Copy the following to cite this article: Alshrari A. S, Hudu S. A, Jimoh A. O, Badr B. M. Prevalence of Genital Herpes: Insights from Outpatient Clinic Patients. Biomed Pharmacol J 2024;17(4). |
| Copy the following to cite this URL: Alshrari A. S, Hudu S. A, Jimoh A. O, Badr B. M. Prevalence of Genital Herpes: Insights from Outpatient Clinic Patients. Biomed Pharmacol J 2024;17(4). Available from: https://bit.ly/4203qJJ |
Introduction
Herpes simplex virus (HSV) is a double-stranded DNA genera that contains two members, HSV-1 and HSV-2 cause persistent infection with recurrent lesions 1. It manifests as groups of vesicles on an erythematous base and is caused by DNA viruses in the Herpesviridae family. A type called HSV-1 causes mainly oral infections like herpes labialis or cold sores and another, HSV-2 mostly genital ones.2. The lesions of HSV-1 and HSV-2 are indistinguishable per Clinique, however, both are primarily spread through direct contact. After an initial infection, the virus is latent in the spinal dorsal root ganglia that innervates the skin of even a single dermatome 3, 4. In all cases of recurrence, the virus travels down nerves to infected areas on or around the skin and mucous membrane where it multiplies, producing large lesions. Upon completion of each episode, the virus remains latent and does so for life.
The health burden of HSV-2 infection differs per region, with varied associated risk factors among different populations. Almost 700 million people are living with HSV-2 worldwide and the prevalence is highest in some parts of Africa and the Americas whereas Asia has lower rates 5, 6. The Centers for Disease Control estimates that too many people, approximately one in six sexually active adults suffer from genital herpes most of them women 7. High rates are also observed in parts of sub-Saharan Africa, where there is a high prevalence of HIV with up to 80% of HIV-positive teenagers from South Africa being seropositive for HSV-2, as well as 20% of their corresponding healthy controls 8, 9. The average HSV-2 seropositivity rate among antenatal clinic attendees in Africa was >40%, while the prevalence was 60–95% amongst female sex workers, especially those from sub-Saharan districts 10.
HSV-2 prevalence varies markedly by individual characteristics such as gender, age, sexual behaviour, marital status, education, and race 11. Most incident genital herpes infections do not present as recognized clinical syndromes but rather are experienced as subclinical or unrecognized 12. Indeed, asymptomatic infection is perhaps the most important factor in maintaining virus circulation; many cases of genital herpes are transmitted by individuals who have no clinical awareness of their infected status 13. Nigeria is a country in sub-Saharan Africa where the prevalence of both HSV-2 and HIV infection is high 14. A study in Lagos reported a 59% seroprevalence among female sex workers and one from Port Harcourt, Rivers State recorded as high as 58.9% for HSV-2 IgG antibodies 15, 16. There is a paucity of data on the seroprevalence of HSV-2 in Sokoto. This study specifically investigated the seroprevalence of HSV-2 infections in febrile patients attending Specialist Hospital, Sokoto.
Materials and Methods
A cross-sectional study was conducted at the outpatient clinic of the Specialist Hospital, Sokoto. In order to obtain a population-representative sample blood samples were harvested from every second patient with an acute febrile episode.
Study Population
Patients who visited the outpatient clinic between April and August of 2023 with a fever, which was defined as a body temperature that was higher than 37 degrees Celsius, were included in the study. These individuals came from a variety of various backgrounds and represented a range of ages and genders.
Data Collection
Information on socio-demographic variables and probable risk factors for HSV-2 infection was collected using a semi-structured questionnaire.
Sample Collection and Processing
As part of the experiment, a laboratory technologist extracted three to four millilitres of venous blood from each participant and placed it in EDTA vials. After that, the samples were centrifuged at a speed of 2,500 revolutions per minute for five minutes to separate the plasma, which was then kept at a temperature of -20 degrees Celsius until it was analysed.
Sample Analysis
Plasma samples were used for enzyme-linked immunosorbent assay (ELISA) kits purchased from Diagnostic Automation (Cortez Diagnostics Inc. USA) as per the manufacturer’s instructions. In both of human plasma, the assay detected IgG antibodies specific for HSV-2.
Assay Procedure
A 96-well plate was prepared by adding a dilution of the negative control, the positive control, the calibrator, and each plasma sample, each of which was 100μL in volume. Additionally, one well was reserved as a reagent blank from the experiment. After thirty minutes of incubation at room temperature, the plate was washed three times before beginning the next step. After that, 100 microlitres of enzyme conjugate was introduced into every well, placed in an incubator at room temperature for 15 minutes, and then washed once more. Following this, 100 microlitres of TMB substrate was introduced onto the plate, and it was then left to incubate for a further 15 minutes. The reaction was terminated by adding 100 microlitres of 2M hydrochloric acid, and the optical density (OD) was measured over five minutes using an ELISA plate reader at a wavelength of 450 nm.
Calculation and Interpretation of Results
The cutoff optical density (OD) value was determined by multiplying the OD of the calibrator by a factor (f) specified on the calibrator vial (f = 0.5). The IgG index for each sample was then calculated by dividing its OD value by this cutoff. An IgG index of ≤ 0.90 was classified as seronegative, while an index between 0.91 and 0.99 was considered equivocal, necessitating retesting. In contrast, an IgG index of ≥ 1.00 was regarded as seropositive.
Data Analysis
SPSS software version 16.0 (SPSS Inc., Chicago, USA) was used for statistical analysis. To assess for associations between variables, multivariate regression analysis was conducted with a 95% confidence interval. Statistical significance was considered at a p-value of ≤ 0.05.
Results
A total of 184 plasma samples obtained from febrile patients were evaluated for HSV-2 IgG, and 100 (54.3%) were positive as determined by inhibition assay. three-quarters (95% CI = 80.6–91.4) were aged between 18–37 years, with the age group 23–27 years most commonly affected at 31.5% (58/184). HSV-1 IgG was detected in all age cohorts, with the fewest numbers detected in individuals aged ≤53 years (n = 6). All six of the patients, aged 43 to 47, tested positive for the virus. The seroprevalence of HSV-2 IgG did not differ significantly by age (p = 0.729) (Table 1). It includes more females (122/184) than males (62/184). Of women, 66.3% (122/184) were enrolled with fever, nearly twofold compared with men. Men had a higher prevalence of HSV-2 IgG than women (67.7%, 42/62 vs. 47.5%, 58 /122, P < 0.0001). Although seroprevalence between groups was not significantly different (p = 0.066), male patients were 2.3 times more likely to be infected with HSV-2 than females (OR 2.317; CI 95% = 0.937-5.730).
Occupational status Majority of the patients attending the Specialist Hospital, Sokoto are married 110/184 (60.1%). On the other hand, a significantly higher proportion of HSV-2 IgG seropositivity (65%, 52/80) was recorded among single individuals than for married ones (48.3%; 54 out of 112); however, this difference did not reach a statistical significance level via chi-square test, p =.141). Employed patients had the highest seroprevalence of HSV-2 IgG (68.2%, 30/44) and self-employed individuals recorded the lowest prevalence rate (55.1%, 54/98), but there was no statistically significant difference between all occupations groups adjusted for an age variable.
Table 1: Seroprevalence of HSV-2 about age among patients presenting with fever at the Specialist Hospital, Sokoto
| Age group (years) | Total | Positive (%) | Negative (%) | p-value |
| 18–22 | 22 | 26(59.1) | 18(40.9) | p = 0.729 |
| 23–27 | 58 | 30(51.7) | 28(48.3) | |
| 28–32 | 42 | 22(52.4) | 20(47.6) | |
| 33- 37 | 14 | 6(42.9) | 8(57.1) | |
| 38–42 | 14 | 8(57.1) | 6(42.9) | |
| 43–47 | 6 | 6(100.0) | 0(0) | |
| 48–52 | 4 | 2(50.0) | 2(50.0) | |
| ≥ 53 | 2 | 0(0) | 2(100.0) | |
| Total | 184 | 100(54.3) | 84(45.7) |
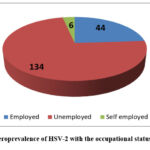 |
Figure 1: Seroprevalence of HSV-2 with the occupational status of patientsClick here to View Figure |
Concerning knowledge of HSV-2, results showed that 91.3% (168/184) patients did not know the virus at all and only 8.7% or n =16/184 ever heard of genital herpes. Seventy-five percent (12/16) of 19 who were aware had HSV-2, compared to over half of the total population at risk unaware124(88/168), P = 0.220. Three patients had heard about HSV-2 during hospital visits, to a school lecture and 1 patient learnt from life.
In terms of possible risk factors for HSV-2 infection, the analysis associated with HSV-2 in this study was (p> 0.05). The seroprevalence of HSV-2 was found in 60% (18/30) and 51.6% (32/62), between patients not sexually active and those that were engaged to be sex-active, respectively-p = 0.449 Fifty-two percent (32/61) of those with only one partner and 100% of the patient who reported multiple partners were seropositive, respectively (p =.538). Of the 10 (19.6%) patients who reported placing only partial vaginal penetration, five seroconverted within six months of potential exposure and all were positive for HSV-2 IgG at their follow-up visit; two others had been positive, to begin with, while three stayed non-reactive in both analyses.
Discussion
Over half (100/184; 54.3%) of the patients presenting with fever were seropositive for HSV-2 in this study. This rate is consistent with 59% and 47.3% seroprevalence rates, found in previous Nigeria-based studies 17, 18. This, however, is lower than the seroprevalence rates of 87% and 77.8% reported in Jos and Enugu, Nigeria respectively19, 20, but higher than the 16.5 % recorded from research done in the United States 21. The number of seropositive patients is indicative that many are probably unaware of their infection because HSV-2 infections often remain asymptomatic. They are consequently a population with increased susceptibility to HIV because HSV-2 infection can be expected. HSV-2 establishes a lifelong infection with periods of recurrent disease, leaving such patients reservoirs for the virus. The presence of anti-HSV antibodies helps diagnose persons who are carrying the infection. Secondly, patients co-infected with HIV might develop more frequent recurrent fevers caused by HSV-2 reactivation. These patients find it challenging to cope with this condition, which manifests in a combination of medical, psychological, and social symptoms.
In this study, the highest seroprevalence of HSV-2 was found to be among those aged 43–47 years, which is to some degree like another finding in a Jos-based study as well, where participants within an age range of 51–60 had the highest prevalence. As observed in other countries and previous studies conducted in Nigeria, the prevalence of HSV-2 increases with age. 9, 22. Similarly, based on research in Brazil. 5, Croatia 23, India 24, Morocco, and Sri Lanka 25, age is strongly associated with a greater prevalence of HSV-2. Moreover, the seroprevalence was found higher among males than females in this study, consistent with previous reports. This could be related to more frequent sexual activity or a higher number of sex partners among men. In contrast, other research has shown increased HSV-2 in women relative to men.
We also found an even higher seroprevalence among single patients than married ones again, in line with studies from the United States26. This is the case in Kenya and Nigeria, where high sexual activity outside of marriage with a mix of one or more sex partners per month increases exposure to commercial workers27. Patients self-identifying as nonsexual active were more likely to be seropositive for HSV-2 than those with sexual activity; all the other patients, save one person who had several partners, tested negative. Many studies have proposed that sexual transmission of the virus occurs, and there is an association between having more sex partners and having higher HSV-2 seropositivity28-30. Seroconversion was higher in unprotected cases compared to those who did, contrary to previous reports on the use of protection.
Conclusion
Conclusions Our results since 54.3% of patients reacted positively in the serology test with no signs or symptoms related to infection detected during physical examination. They are therefore silent virus carriers. HSV-2 infection was not significantly associated with the socio-demographic background and predisposing risk factors assessed in the study. The public health implications of HSV-2 in Nigeria are highlighted by the results, given that anti-HSV-2 testing is not generally available and infants would be at high risk from neonatal transmission with possible complications during delivery. Public awareness and information on genital herpes, the many ways by which it may be transmitted and its public health importance based on high seroprevalence of HSV-2 in this study is necessary. Future research should focus on whether patients with frequent fever need to be investigated for HSV-2 antibodies and such studies can reveal findings that are statistically significant if done in a larger study population. As the treatment of HSV-2 is lifelong and there is no known cure, primary prevention plays a crucial role in controlling it.
Acknowledgement
The author wishes to thank Zarqa University, Jodan for providing support in publishing this manuscript.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The author(s) do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
The ethical approval to conduct this research was obtained from Specialist Hospital, Sokoto Research Ethics Committee (SHS/SUB/145/Vol2)
Informed Consent Statement
Informed consent was obtained from all patients before experimentation. The protection of human subjects’ privacy rights should always be maintained.
Clinical Trial Registration
This research does not involve any clinical trials
Author Contributions
Ahmed Subeh Alshrari: Conceptualization, Methodology, Writing – Original Draft.
Shuaibu Abdullahi Hudu: Data Collection, Analysis, Writing – Review & Editing.
Abdulgafar Olayiwola Jimoh: Visualization, Supervision, Project Administration.
Bahaa Mohammed Badr: Funding Acquisition, Resources, Supervision.
References
- Maris AS, Tao L, Schmitz JE: Herpes simplex viruses. Molecular Medical Microbiology: Elsevier. 2024; 2559-75.
CrossRef - James SH, Kimberlin DW: Herpes simplex virus infections. Remington and Klein’s Infectious Diseases of the Fetus and Newborn Infant. 2025:745-64. e4.
CrossRef - Canova P, Charron A, Leib D: Models of Herpes Simplex Virus Latency. 2024; 16:747-.
CrossRef - Alshrari A, Hudu S, Elmigdadi F, Imran M: The Urgent Threat of Clostridioides difficile Infection: A Glimpse of the Drugs of the Future, with Related Patents and Prospects. 2023; 11:426-.
CrossRef - de Oliveira Bonfim F, Villar L, Croda J, Pereira J, Guimarães A, da Silva S, CC MG, Leonardo L, de Rezende Romeira G, Cesar G: High silent prevalence of human herpesvirus 1 (HSV-1) infection affecting the indigenous reservation of the municipality of Dourados, Central-West Brazil. BMC Infectious Diseases. 2024; 24:700-.
CrossRef - Chaiyakunapruk N, Lee SWH, Kulchaitanaroaj P, Rayanakorn A, Lee H, Looker KJ, Hutubessy R, Gottlieb SL: Estimated global and regional economic burden of genital herpes simplex virus infection among 15–49 year-olds in 2016. BMC Global and Public Health. 2024; 2:42.
CrossRef - Johnston C, Wald A. Genital Herpes. JAMA. 2024;332(10):835-6..
CrossRef - Asare K, Ngcapu S, Osman F, Mindel A, Naicker N, Khanyile M, Karim SSA, Tomita A, Garrett N: Incidence of herpes simplex virus type 2 positivity among women living with human immunodeficiency virus in South Africa. International journal of STD & AIDS. 2024; 35:58-66.
CrossRef - Oshun PO, Mutiu B, Bode-Sojobi I: Prevalence and Risk Factors for Herpes Simplex Virus Type 2 Infections Among HIV Infected Individuals in Lagos. Annals of Tropical Pathology. 2024; 15:17-22.
- Mcdonald U, Nyawale H, Kajura A, Mujuni F, Chibwe E, Silago V, Msemwa B, Minja C, Daffa Z, Karim M: High Seropositivity of Markers of Viral Infections among Women with Unfavorable Pregnancy Outcomes in Mwanza, Tanzania: The Urgent Need for Control Interventions. The East African Health Research Journal. 2023; 7:25-31.
CrossRef - Casto AM, Johnston C, Stanberry LR: Human Herpesviruses: Herpes Simplex Virus Types 1 and 2. Viral Infections of Humans: Epidemiology and Control: Springer, 2023. 1-48.
CrossRef - Looker K, Johnston C, Welton N, James C, Vickerman P, Turner K, Boily M, Gottlieb S: The global and regional burden of genital ulcer disease due to herpes simplex virus: a natural history modelling study. BMJ Global Health. 2020; 5:e001875-e.
CrossRef - Van Wagoner N, Qushair F, Johnston C: Genital herpes infection: progress and problems. Infectious Disease Clinics, 2023; 37:351-67.
CrossRef - Nwadike VU, Osinupebi P, Ojo OY, Imhonopi GB, Oyesola OA, Elikwu CJ, Oladosu WO. Seroprevalence and risk factors of Herpes Simplex Virus Type 2 amongst patients’ attending retroviral clinic in Federal Medical Center Abeokuta, south-west Nigeria. Annals of Ibadan Postgraduate Medicine. 2020;18(1):37-43.
- Aravantinou M, Plagianos M, Kokogho A, Adebajo S, Nowak RG, Shoyemi E, Ekeh C, Lombardi K, Peel SA, Baral SD: Herpes Simplex Virus Type 2 Prevalence and Association with Inflammatory Cytokines Among Sexual and Gender Minorities Living With and Without HIV-1 from Lagos, Nigeria. AIDS research and human retroviruses. 2023; 39:485-94.
CrossRef - Shihata Hassan SA: Prevalence of Herpes Simplex Virus in Pregnant Women in Ismailia City. Advances in Environmental and Life Sciences. 2023; 4:43-51.
- Oripelaye MM, Olanrewaju FO, Ajani AA, Akinboro AO, Enitan AO, Oninla OA: Seroprevalence of varicella-zoster virus among people living with human immunodeficiency virus in Ile-Ife, Nigeria: A cross-sectional study. Journal of Clinical Sciences. 2024; 21:14-9.
CrossRef - Onu EN, Ekuma UO, Judi HK, Ogbu O, Okoro N, Ajugwo GC, Akrami S, Okoli CS, Anyanwu CN, Saki M: Seroprevalence of antibodies to herpes simplex virus 1 and 2 in patients with HIV positive from Ebonyi State, Nigeria: a cross-sectional study. BMJ open. 2023; 13:e069339.
CrossRef - Agabi Y, Banwat E, Mawak J, Lar P, Dashe N, Dashen M, Adoga M, Agabi F, Zakari H: Seroprevalence of herpes simplex virus type-2 among patients attending the Sexually Transmitted Infections Clinic in Jos, Nigeria. Journal of Infection in Developing Countries. 2010; 4:572-5.
CrossRef - Ojinmah UR, Nnoruka E, Ozoh G, Onyekonwu C, Aguwa E: Herpes simplex virus type 2 infection among females in Enugu, Enugu state. Nigerian Journal of Medicine. 2012; 21:394-403.
- McClymont E, Tan DH, Bondy S, Albert A, Coutlée F, Lee M, Walmsley S, Ogilvie G, Money D: HSV-2 infection and HPV incidence, persistence, and precancerous lesions in a cohort of HPV-vaccinated women living with HIV. International journal of STD & AIDS. 2023; 34:402-7.
CrossRef - Yahaya I, Joshua F: Prevalence of herpes simplex virus and associated risk factors among female students of natural and applied science, Nasarawa state university, Keffi. GSC Advanced Research and Reviews. 2023; 16:019-27.
CrossRef - Vilibic-Cavlek T, Belamaric M, Ferenc T, Navolan D, Kolaric B, Milasincic L, Antolasic L, Vilibic M, Lukunic A, Bogdanic M: Seroepidemiology of Herpes Simplex Viruses Type 1 and 2 in Pregnant Women in Croatia. Medicina. 2024; 60:284-.
CrossRef - Gopinath R, Sundaram AM, Dhanasezhian A, Arundadhi M, Thangam GS: Seroprevalence of Various Viral Diseases in Tamil Nadu, India. Journal of Global Infectious Diseases. 2023, 15:144-8.
CrossRef - Cowan FM, French R, Mayaud P, Gopal R, Robinson N, De Oliveira SA, Faillace T, Uusküla A, Nygård-Kibur M, Ramalingam S: Seroepidemiological study of herpes simplex virus types 1 and 2 in Brazil, Estonia, India, Morocco, and Sri Lanka. Sexually transmitted infections. 2003; 79:286-90.
CrossRef - Bauer GR, Khobzi N, Coleman TA: Herpes simplex virus type 2 seropositivity and relationship status among US adults age 20 to 49: a population-based analysis. BMC infectious diseases. 2010; 10:1-10.
CrossRef - Harfouche M, AlMukdad S, Alareeki A, Osman AM, Gottlieb SL, Rowley J, Abu-Raddad LJ, Looker KJ. Estimated global and regional incidence and prevalence of herpes simplex virus infections and genital ulcer disease in 2020: Mathematical modeling analyses. medRxiv. 2024; 2024-06..
CrossRef - Whittles LK, Galiwango RM, Mpagazi J, Tobian AA, Ssekubugu R, Jackson J, Peer AD, Kennedy C, Nakalanzi M, Ndyanabo A: Age patterns of HSV-2 incidence and prevalence in two Ugandan communities: a catalytic incidence model applied to population-based seroprevalence data. The Journal of Infectious Diseases. 2023; 228:1198-207.
CrossRef - Omori R, Abu-Raddad LJ: Sexual network drivers of HIV and herpes simplex virus type 2 transmission. Aids. 2017; 31:1721-32.
CrossRef - Alsuliman T, Musiu P, Stocker N, Desnica L, El-Cheikh J, Sestili S, Srour M, Marjanovic Z, Alrstom A. Sexually transmitted infections in the context of haematological malignancies. The Lancet Haematology. 2024;11(10):e792-802.
CrossRef








