Namir I. Mohammed* and Ahmed Q. Al-Awadi
and Ahmed Q. Al-Awadi
Department of Pathology and Poultry, Disease College of Veterinary Medicine / University of Baghdad, Baghdad, Iraq.
Corresponding Author E-mail:namer.i@uokerbala.edu.iq
DOI : https://dx.doi.org/10.13005/bpj/3061
Abstract
This study evaluated zinc oxide nanoparticles (ZnO-NPs) as immunological adjuvants against Staphylococcus lugdunensis. Fifty male rats (8–10 weeks old) were divided into five groups. Group 1 received sterile saline (negative control), Group 2 was infected with S. lugdunensis (positive control), Group 3 was immunized with sonicated S. lugdunensis antigens, Group 4 received sonicated antigens loaded on ZnO-NPs, and Group 5 received ZnO-NPs only. Serum levels of IL-10 and IgG were measured 28 days post-immunization, and internal organs (heart, kidney, and lung) were examined histopathologically at 7 and 21 days post-infection. Groups immunized with antigens (Groups 3 and 4) showed significantly higher IL-10 and IgG levels compared to controls. Histopathological findings revealed severe vascular congestion in the heart, mild glomerular atrophy with edema in the kidney, and lung hemorrhages in infected groups, while granulomatous lesions were only found in Groups 2 and 3. Immunization with sonicated antigens alone or combined with ZnO-NPs improved immune response and reduced tissue damage. The most effective immune stimulation and protection were observed in the group receiving ZnO-NP-loaded antigens, demonstrating their potential as adjuvants to enhance immune defense and mitigate the effects of S. lugdunensis infection.
Keywords
Immune Response; Sonicated Ag; Staphylococcus lugdunensis; ZnO-NPs
Download this article as:| Copy the following to cite this article: Mohammed N. I, Al-Awadi A. Q. Potential of Zinc Oxide Nanoparticle as Adjuvant Therapy Against Staphylococcus lugdunensis by Modulating Immune Response. Biomed Pharmacol J 2024;17(4). |
| Copy the following to cite this URL: Mohammed N. I, Al-Awadi A. Q. Potential of Zinc Oxide Nanoparticle as Adjuvant Therapy Against Staphylococcus lugdunensis by Modulating Immune Response. Biomed Pharmacol J 2024;17(4). Available from: https://bit.ly/3ZXOyZD |
Introduction
Zinc oxide nanoparticles (ZnO-NPs) are the second most prevalent metal oxide besides iron. They are cost-effective, safe, and easy to synthesize.1 (ZnO-NPs) is well recognized for its extensive application as a chemical additive produced from zinc, which is used in both industrial and daily chemical processes. However, the progress of nanotechnology has resulted in the rising adoption of ZnO-NPs as a good alternative to ZnO materials in several fields, such as industrial manufacturing, biomedicine, clinical treatment, food processing, and environmental management 2. These materials are synthesized from ZnO using several techniques, including physical approaches3, chemical methods4,5, and biological template methods. Environmental synthesis technology enables the large-scale manufacture of ZnO nanoparticles without any additional contaminants6,7,8,9,10. The green synthesis NPs approach has been expanded to include the application of microbes, fruits, bacteria, fungi, algae, and plants in the manufacture of nanoparticles. This technique possesses intrinsic qualities of environmental benignity, economic efficiency, biocompatibility, safety, and sustainability11.
The primary nanoparticles used are ZnO-NPs. Because of their capacity to absorb UV radiation, they are often used in sunscreen compositions12,13. Furthermore, ZnO-NPs are utilized as a photoconductive additive in electronics, as well as as an antibacterial and anti-yeast agent in the food and packaging industries14. ZnO-NPs also show promise as potentially useful therapeutic tools for neurological disorders and novel anti-tumor therapeutic agents15. Novel antileishmanial agents based on ZnO nanoparticles were designed as an alternative choice to traditional drugs for Leishmania tropica parasites16.
Despite its rising prevalence, pathogenicity, and antibiotic resistance, no vaccines currently exist to prevent S. lugdunensis infections. This represents a significant gap in the available prophylactic measures. Innovative approaches are needed to address this gap and reduce reliance on antibiotics for managing infections caused by this pathogen. One such approach involves immunization using sonicated S. lugdunensis antigens carried by zinc oxide nanoparticles (ZnO-NPs) as an adjuvant. ZnO-NPs have demonstrated antimicrobial and immune-modulating properties, making them a promising candidate for enhancing antigen-specific immune responses 17,18,19. By combining bacterial antigens with ZnO-NPs, this study seeks to develop an effective immunization strategy against S. lugdunensis infections, offering a novel therapeutic and preventive avenue for combating this emerging pathogen.
Widely recognized as a potent human pathogen, the behavior of S. lugdunensis closely mimics that of Staphylococcus aureus, exhibiting more virulence than other CoNS agents20. Staphylococcus lugdunensis is capable of inducing a wide range of infections, spanning from localized to systemic disorders. It can develop biofilms, which in turn leads to a variety of infections associated with foreign bodies, such as catheter-related bacteremia, bone infections, and joint infections21. Severe infective endocarditis22 has also been documented in cases of skin and soft tissue infections23.
Staphylococcus lugdunensis has emerged as a significant opportunistic pathogen within the Staphylococcus genus, causing severe infections such as wound and bloodstream infections. Unlike other species such as Staphylococcus aureus and Staphylococcus epidermidis, the attention given to S. lugdunensis has historically been limited, despite its growing clinical relevance. Recent studies in Iraq have highlighted the pathogenicity, antibiotic resistance, and prevalence of Staphylococcus lugdunensis. 24 reported the isolation of methicillin-resistant S. lugdunensis (MRSL) from clinical samples, identifying high resistance rates to β-lactam antibiotics, including oxacillin (46.6%) and ampicillin (73.3%), with the mecA gene detected in 40% of isolates. Similarly, 25 found that S. lugdunensis isolates exhibited 100% resistance to methicillin and oxacillin in samples collected from dental staff. These findings highlight the bacterium’s growing resistance to conventional antibiotics, presenting significant challenges for clinical management. Furthermore, its presence has been documented in diverse clinical samples in Iraq, including burns, skin, pus, and blood 26,27 The prevalence of S. lugdunensis in healthcare and community settings indicates its adaptability and pathogenic potential. Beyond its antibiotic resistance, S. lugdunensis exhibits additional characteristics that complicate treatment.27 identified biofilm-associated genes (icaA and icaD) in S. lugdunensis, underscoring its ability to form biofilms, which enhance resistance to antibiotics and immune evasion. Moreover, the study by28 demonstrated the bacterium’s high resistance to environmental stressors, such as mercury and chromium, and its ability to thrive in polluted environments, reflecting its resilience and survival adaptability.
Materials and Methods
The steps for Staphylococcus lugdunensis isolate are mentioned according to 29,30. The bacterial cultures used in the current study were obtained from the Department of Biology, College of Science, University of Baghdad, Iraq. Vitec and PCR Assay confirmed The Staphylococcus. Lugdunensis
Preparation of Staphylococcus. Lugdunensis Antigens
This was prepared as follow31, briefly, Staphylococcus lugdunensis cultured on nutrient agar was incubated at 37 °C for 24 hrs., harvested in PBS (pH7.2), centrifuged at 3000 rpm (4°C/30 minutes), then the precipitate was washed three times with PBS, re-suspended in PBS in a universal tube, placed in the ultrasonicator device (at 12 Peak for 2 minutes intervals for 30 minutes in a cold environment (ice ). The sonicated suspension was centrifuged at 10,000 r.p.m. for 30 minutes in a cooled centrifuge. The resulting liquid was then filtered using a Millipore filter with a pore size of 0.22 micrometers. The supernatant was then analyzed using Gram stain and cultured on blood agar to verify the sterility of the antigen. Quantification of the antigen using the Biuret technique revealed a total protein concentration of 3 mg/ml, classifying it as soluble sonicated S. lugdunensis antigen (Sonicated Ag).
Preparation of Zinc Oxide Nanoparticles
The synthesis of zinc oxide nanoparticles was conducted using biological methods at the Department of Biotechnology, College of Science, University of Baghdad, Iraq. The Allium porrum leaves were collected from the cultivation site in Kerbela, Iraq, rinsed with tap water, and thoroughly desiccated. The desiccated foliage was pulverized into a fine powder. Next, 250 grams of the powder were combined with 500 ml of deionized water. The combination was then stirred for one hour and then stored at 4°C for 24 hours. Next, the mixture was centrifuged at 8,000 r.p.m. for 10 minutes. The supernatant was promptly collected and placed in the refrigerator for the subsequent procedure. 200 cc of the prior preparation was combined with 20 g of zinc acetate and agitated overnight in a shaker. Next, the mixture was briefly centrifuged to gather the nanoparticles. After being rinsed twice with 5 ml of deionized water, the precipitated nanoparticles were centrifuged at 8,000 r.p.m. for 5 minutes, recovered, and transferred to a sterile Petri plate and maintained at 37°C for 24 hours to dry. Finally, the end product was characterized to verify the incorporation of nanoparticles.
Preparations for in vivo administration
Amounts of ZnO-NPs (size of nanoparticle was 65.49 nm), 0.312 mg ZnO-NPs was added to 5 ml of S. lugdunensis antigen suspension. The resulting mixtures were gently stirred at 37°C on a sonicated water path device.
In vivo experimental Design
Forty healthy white male rats, ages ranging between 8-10 weeks, were randomly allocated into five groups and administered the treatments as follows:
Group 1 (n=5): rats injected with 0.3 ml of sterile normal saline serving as a negative control group.
Group 2 (n=10): rats injectedp with S. lugdunensis (1.5×108 Cell/ml) serving as positive control group.
Group 3 (n=10): rats immunized s.c. with 0.3 ml (3mg/ml) of whole sonicated lugdunensis antigens, 2 doses with 2 weeks intervals, then infected with S. lugdunensis (1.5×108 cells/ml).
Group 4 (n=10): rats immunized s.c. with 0.3ml (3mg/ml) of whole sonicated lugdunensis antigens uploaded on ZnO nanoparticles, 2 doses with 2 weeks intervals, then infected with S. lugdunensis.
Group 5 (n=5): rats injected s.c. with 0.3 ml of ZnO-NPs, serving as an additional negative control group.
At day 28 post-immunization, serum was collected for detection of IgG and IL-10, according to the manufacturer’s suggested protocol (Sunlong Medical Company /Catalogue Number: IgG/EL0020Ra; Sunlong Medical Company /Catalogue Number: IL10/ EL0032Ra).
At day 30 post-immunization, the rats were infected with S. lugdunensis given i.p. Five rats from each group were sacrificed at days 7 and 21 post-infection, and samples from internal organs (heart, kidney, and lung) were taken for histopathology.
Statistical Analyses
A datasheet of IBM SPSS version 26.0 was used to tabulate the data and conduct the statistical analysis. Data are expressed as mean and standard errors of continuous variables. Statistical comparison was performed using an analysis of variance (ANOVA) test to identify significant differences, followed by an evaluation of the least significant difference (LSD). A p ≤0.05 was considered significant.
Results
As shown in Figure 1, the PCR approach detected the Fbl gene of S. lugdunensis to confirm the bacterial species.
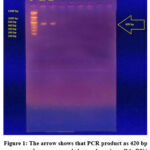 |
Figure 1: The arrow shows that PCR product as 420 bp represented on agarose gel electrophoresis, well 1: DNA ladder marker, well 1 and 2 represented positive samples |
Figures 2 and 3 revealed high levels of IgG at day 28 post-immunization and day 7 post-infection in the 2nd and 3rd groups, respectively. In addition, the level of IL-10 also showed a significant increase in the same groups. This suggests that exposure to sonicated antigen (group 3) or the combination of zinc oxide and sonicated antigen (group 4) can lead to an increase in IgG and IL-10 levels.
The group 5 (ZnO-NPs) showed a decrease in the levels of IgG and IL-10 at day 7 post-infection compared with day 28 post-immunization (before infection). The sonicated antigen could potentially enhance the immune response by increasing its presentation efficiency to the immune cells. This can lead to a robust activation and proliferation of B cells, which are responsible for producing IgG antibodies. As a result, the levels of IgG and IL10 in the blood were higher in the group exposed to the sonicated antigen compared to the other groups.
When the sonicated antigen was delivered in conjunction with ZnO-NPs, it helped maintain higher levels of IL-10 compared to ZnO-NPs alone. The sonicated antigen generated from S. lugdunensis partially offset the immunosuppressive effects of ZnO-NPs. ZnO-NPs appeared to interfere with the immune system’s typical response since they inhibited the production of the anti-inflammatory cytokine IL-10 and IgG. However, this immunosuppressive impact of the ZnO-NPs was partially offset by the sonicated antigen. Compared to the group that received ZnO-NPs alone, higher levels of IL-10 were achieved by co-administering the sonicated antigen with the ZnO-NPs. it implies that the sonicated antigen blunts the ZnO-NPs’ immunomodulatory effects.
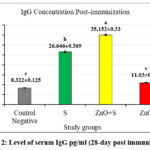 |
Figure 2: Level of serum IgG pg/ml (28-day post immunization) |
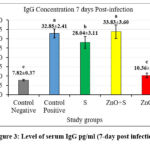 |
Figure 3: Level of serum IgG pg/ml (7-day post infection) |
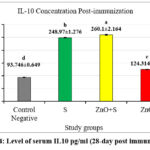 |
Figure 4: Level of serum IL10 pg/ml (28-day post immunization) |
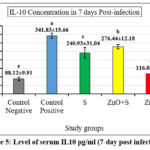 |
Figure 5: Level of serum IL10 pg/ml (7-day post infection) |
Histopathological examination
Histopathological examination on day 7 post-infection in the positive control group revealed that the heart showed severe congestion of blood vessels and mild hemorrhage (Figure 6a). At the same time, the kidney showed infiltration of mononuclear cells (MNCs) in the interstitial tissue and mild atrophy of the glomerular tuft with edematous fluid in the Bowman space (Figure 6b). The heart showed at day 7 post-infection that the group 3 rats immunized with the sonicated Ag showed normal cardiac tissue (Figure 7a), and the kidney showed congestion of blood vessels and MNCs infiltration (Figure 7b). The lung revealed mild MNCs infiltration in the parenchyma and severe perivascular cuffing of MNCs (Figure 7c). At the same time point, the group 4 rats given the sonicated Ag+ZnO-NPs showed normal architecture of the heart (Figure 8a) and slight peri-glomerular MNCs infiltration (Figure 8b). The lungs were characterized by mild thickening in the alveolar walls and mild emphysema in addition to perivascular and peribronchiolar cuffing of MNCs (Figure 8c).
The histological sections from the hearts of the positive controls (group 2) at day 21 post-infection showed congested blood vessels and mild MNCs infiltration in the cardiac interstitium (Figure 9a). The kidney showed peri-glomerular and interstitial infiltration with MNCs (Figure 9b). The lung showed severe MNC infiltration in the alveolar and peri-bronchial interstitium (Figure 9c). In group 3 rats immunized with the sonicated Ag at day 21 post-infection, the heart showed congestion of blood vessels and mild MNC interstitial infiltration (Figure 10a). The kidney showed congestion of blood vessels, MNCs infiltration, and focal granulomatous lesions in the kidney parenchyma (Figure 10b). The lung showed severe MNCs infiltration in the parenchymal interstitium (Figure 10c).
In group 4 rats immunized with sonicated Ag+ZnO-NPs, the heart showed a normal architecture (Figure 11a). The kidney showed MNCs adjacent to the glomeruli (Figure 11b), while the lung showed MNCs infiltration and focal granulomatous lesions in the lung parenchyma, as well as cellular debris in the bronchiolar lumina (Figure 11c).
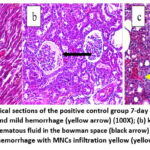 |
Figure 6: Histopathological sections of the positive control group 7-day post infection showed: (a) heart: congestion and mild hemorrhage (yellow arrow) (100X); |
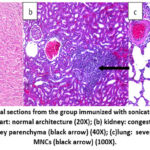 |
Figure 7: Histopathological sections from the group immunized with sonicated Ags group 7 days post-infection showed: (a) heart: normal architecture (20X); (b) kidney: |
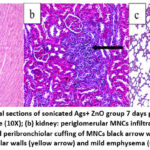 |
Figure 8: Histopathological sections of sonicated Ags+ ZnO group 7 days post-infection showed: (a) heart: normal architecture (10X); (b) kidney: periglomerular MNCs infiltration (black arrow) (100X); |
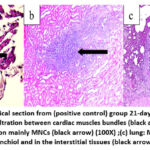 |
Figure 9: Histopathological section from (positive control) group 21-day post-infection showed (a) heart: mild MNCs infiltration between cardiac muscles bundles (black arrow) (40X); (b) kidney: |
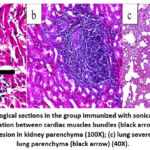 |
Figure 10: Histopathological sections in the group immunized with sonicated Ags showed: (a) heart: mild MNCs filtration between cardiac muscles bundles (black arrow) (40X); |
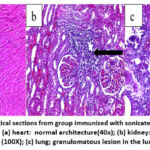 |
Figure 11: Histopathological sections from group immunized with sonicated Ags+ZnO-NPs), 21-day post-infection showed: (a) heart: normal architecture(40x); (b) kidney: |
Discussion
When harmful invaders are detected, the immune system responds by either neutralizing them or eliminating them 32. Nanoparticles (NPs) derived from plant extract have emerged as a promising approach in the field of nanotechnology and nanomedicine, particularly for their ability to modulate the immune response. Moreover, immune stimulation after using nanoparticles as adjuvant can be considered a safe and effective approach to stimulate IgG production, IgG playing a crucial role in the host’s defense mechanism. Plant-derived nanoparticles can stimulate IgG production owing to their inherent biocompatibility and ability to be easily recognized by the immune system. Interestingly, many bioactive compounds, such as flavonoids, terpenoids, and alkaloids, have been detected in these nanoparticles, which are capable of interacting with immune cells and triggering their innate and adaptive response. Upon administration of these nanoparticles, T-accessory cells, such as dendritic cells and macrophages, can improve the phagocytosis of vaccine components and increase antigen presentation to Th2 cells, which in turn stimulate B cells to produce IgG33. IgG is known to contribute to the adaptive immune response. During an infection caused by Staphylococcus. lugdunensis, B cells can identify the bacterial antigens and generate specific IgG antibodies to target and inactivate the pathogen. Once bound to the bacteria, IgG designates them for elimination by other components of the innate defense system, such as phagocytic cells and complement. Our study reveals that the use of sonicated S. lugdunensis antigen coupled with Zn-O nanoparticles enhanced the production of specific IgG antibodies. It, in turn, promotes the opsonization and subsequent eradication of S. lugdunensis 33. More importantly, these antigen-bound nanoparticles shorten the time needed for B cell stimulation and increase the production of IgG compared with the rats vaccinated with the sonicated antigen alone.
Zinc oxide nanoparticles (ZnO-NPs) have been shown to have immune-modulatory properties, being able to influence the function of the immune system. Nevertheless, their effects may differ based on their dimensions, configuration, density, and surface characteristics, as well as the experimental parameters and cell types under investigation. A previous pre-clinical study34 demonstrated the effectiveness of using sonicated antigens from S. lugdunensis added with an adjuvant to improve the development of a vaccine that can efficiently induce a robust and protective immune response against S. lugdunensis infection.
The cytokine IL-10 possesses anti-inflammatory properties needed to down-regulate the immune response and reduce excessive inflammation. When exposed to bacterial antigens or inflammatory signals, immune and related cells, such as T cells, macrophages, and dendritic cells, can initiate the synthesis of IL-1035,36, which has a role in modulating the inflammatory response and mitigating tissue damage caused by an exaggerated reaction to the infectious agents. Infection by S. lugdunensis can trigger an immune response characterized by the synthesis of IL-10 37,38. Previous studies have shown that Zn-O nanoparticles intrinsically possess immuno-stimulatory properties, being able to stimulate the molecular pattern recognition receptors, such as the Toll-like receptors (TLRs), on immune cells. This activation can trigger immunological responses and promote the production of inflammatory mediators, including TNF-α and IL639. The present study fits well with the previous findings that a sonicated S. epidermidis preparation induces IL-10 secretion from human mast cells, potentially influencing the immune response40. Other studies have also demonstrated that exposure to sonicated Staphylococcus species can stimulate the release of IL-10 in different cell types41.
Although no research has specifically investigated the effect of combining sonicated S. lugdunensis with ZnO-NPs on IL-10 production, the studies mentioned above performed on other Staphylococcus species indicate that a similar effect may occur. Inflammatory cells may exploit the anti-inflammatory properties of IL-10 to mitigate the reaction to sonicated antigen-bound ZnO-NPs. A further investigation revealed that the co-administration of sonicated S. aureus and ZnO-NPs resulted in increased release of IL-10 from human lung epithelial cells, interpreted as a possible defensive mechanism to the potent immune stimulus42.
In cases of endocarditis due to S. lugdunensis, the congestion of coronary arteries is likely attributable to the inflammatory response induced by the bacterial infection. The resulting inflammatory response causes vasodilation and increased permeability of the coronary vessels, leading to exudate extravasation in the cardiac interstitium. The infection has the potential to harm the endothelial cells, resulting in exacerbated permeability and potential arterial rupture43. The present histological findings suggest glomerular damage, possibly caused by the inflammatory response induced by the S. lugdunensis infection. Itis characterized by infiltration by mononuclear cells, such as lymphocytes and monocytes, into the kidney interstitium44.
S. lugdunensis is known for its capacity to produce many virulence factors, such as toxins and adhesins, that can directly undermine the structural and functional integrity of the pulmonary blood vessels45. Enhanced permeability resulting from this vascular damage induces extravasation of exudate and blood into the adjacent lung tissue, leading to congestion. The collapse of the vascular endothelium can cause a loss of normal vascular tone, which in turn can result in bleeding and hemorrhage in the lung aerial spaces. In the lungs, S. lugdunensis also triggers a strong inflammatory response, marked by the recruitment of mononuclear cells, such as lymphocytes and monocytes, in the site of infection46. In turn, inflammatory cells release several mediators, such as chemokines and cytokines, that can exacerbate vascular dysregulation and recruitment of inflammatory cells. The chronic inflammatory response can lead to lung tissue damage and dysfunction, therefore compromising the gas exchanges. Besides its indirect adverse effects mediated by blood vessel injury, S. lugdunensis also produces several enzymes and toxins that can directly damage lung tissue47, such as hemolysins and leukocidins. These molecules can cause lysis and destruction of host cells, such as alveolar epithelial cells and endothelial cells, as well as disassemble the extracellular matrix and disrupt the alveolar-capillary barrier, further contributing to the development of severe congestion, hemorrhage, and impaired gas exchange in the lungs43.
Conclusion
This study shows the potential of zinc oxide nanoparticles as an effective adjuvant with sonicated Staphylococcus lugdunensis antigens to stimulate immune responses, offer protection against tissue damage from infection, and enhance antigen-based immunization strategies.
Acknowledgment
Authors acknowledge college of veterinary medicine / University of Baghdad. Baghdad / Iraq, college of veterinary medicine / University of Kerbala. Karbala / Iraq.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The author(s) do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics approval
The Animal Care and Use Committee (College of Veterinary Medicine) University of Baghdad reviewed and approved the study. (P.G.2349/ date 24.12.2023).
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials
Author Contributions
Ahmed Q. Al-Awadi: Design and performed the experiments and Data Collection, Analysis
Namir I. Mohammed: Methodology, writing – Review & Editing the manuscript
Reference
- Kalpana VN, Devi Rajeswari V. A review on green synthesis, biomedical applications, and toxicity studies of ZnO NPs. Bioinorganic chemistry and applications. 2018;2018(1): 3569758.
CrossRef - Siddiqi KS, ur Rahman A, Tajuddin N, Husen A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale research letters. 2018; 13:1-3.
CrossRef - Aadim KA, Abbas IK. Synthesis and Investigation of the Structural Characteristics of Zinc Oxide Nanoparticles Produced by an Atmospheric Plasma Jet. Iraqi Journal of Science. 2023 30:1743-52.
CrossRef - Akpomie KG, Ghosh S, Gryzenhout M, Conradie J. One-pot synthesis of zinc oxide nanoparticles via chemical precipitation for bromophenol blue adsorption and the antifungal activity against filamentous fungi. Scientific reports. 2021 15;11(1):8305.
CrossRef - Al-Hraishawi H, Al-Saadi N, Jabbar S. In Vitro Analysis: The Anticancer Activity of Zinc Oxide Nanoparticles from Cinnamomum Verum. Journal of Nanostructures. 2023 1;13(1):146-50.
- Husain WM, Araak JK, Ibrahim OM. Green synthesis of zinc oxide nanoparticles from (Punica granatum L) pomegranate aqueous peel extract. The Iraqi Journal of Veterinary Medicine. 2019 28;43(2):6-14.
CrossRef - Mohd Yusof H, Mohamad R, Zaidan UH, Abdul Rahman NA. Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: a review. Journal of animal science and biotechnology. 2019; 10:1-22.
CrossRef - Alwash A. The green synthesize of zinc oxide catalyst using pomegranate peels extract for the photocatalytic degradation of methylene blue dye. Baghdad Science Journal. 2020 14;17(3):0787-.
CrossRef - Al-Ghareebawi AM, Al-Okaily BN, Ibrahim OM. Characterization of zinc oxide nanoparticles synthesized by olea europaea leaves extract (part L). Iraqi Journal of Agricultural Sciences. 2021 19;52(3):580-8.
CrossRef - Majeed SM, Ahmed ME, Ali IA. Comparison of Sizes of Zinc Oxide Nanoparticles Extracted from Staphylococcus lugdunensis and Berberis vulgaris Plant Extract Against Some Types of Bacteria and Yeast. Journal of Drug Delivery Technology. 2022;12(1):103-7.
CrossRef - Al-Abodi EE. A Review Article: Green Synthesis by using Different Plants to preparation Oxide Nanoparticles. Ibn AL-Haitham Journal for Pure and Applied Sciences. 2023 20;36(1):246-59.
CrossRef - Mohammed YH, Holmes A, Haridass IN, Sanchez WY, Studier H, Grice JE, Benson HA, Roberts MS. Support for the safe use of zinc oxide nanoparticle sunscreens: lack of skin penetration or cellular toxicity after repeated application in volunteers. Journal of Investigative Dermatology. 2019 1;139(2):308-15.
CrossRef - Ahmed ME, Al-Awadi AQ, Abbas AF. Focus on Synergistic Bacteriocin-Nanoparticles Enhancing Antimicrobial Activity Assay. Mikrobiolohichnyi Zhurnal. 2023 21;85(6):95-104.
ttps://doi.org/10.15407/microbiolj85.06.095 - Sánchez-López E, Gomes D, Esteruelas G, Bonilla L, Lopez-Machado AL, Galindo R, Cano A, Espina M, Ettcheto M, Camins A, Silva AM. Metal-based nanoparticles as antimicrobial agents: an overview. Nanomaterials. 2020 9;10(2):292.
CrossRef - Wiesmann N, Tremel W, Brieger J. Zinc oxide nanoparticles for therapeutic purposes in cancer medicine. Journal of Materials Chemistry 2020;8(23):4973-89.
CrossRef - Meaad AG, Ban N. The Effect of Zinc Oxide Nanoparticles (ZnO NPs) on the Viability of Leishmania tropic in Vitro. Iraqi Journal of Science. 2017;58(2A):600-10.
- Sharma P, Jang NY, Lee JW, Park BC, Kim YK, Cho NH. Application of ZnO-based nanocomposites for vaccines and cancer immunotherapy. 2019 26;11(10):493.
CrossRef - Sultan M, Bahjat HO, Ahmed SS, Noomi BS, Abdulhussain NJ, Al-Bayati HH. The promoting role of zinc oxide nano particles (Zo-NPs) enhancing the immunogenic activity of Escherichia coli lipopolysaccharides (ELPS) in vivo. SVU-International Journal of Veterinary Sciences. 2024 1;7(2):36-42.
CrossRef - Liu X, Lin X, Hong H, Wang J, Tao Y, Huai Y, Pang H, Liu M, Li J, Bo R. Polysaccharide from Atractylodes macrocephala Koidz Binding with Zinc Oxide Nanoparticles as a Novel Mucosal Immune Adjuvant for H9N2 Inactivated Vaccine. International Journal of Molecular Sciences. 2024 9;25(4):2132.
CrossRef - Frank KL, Del Pozo JL, Patel R. From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis. Clinical microbiology reviews. 2008;21(1):111-33.
CrossRef - Argemi X, Prevost G, Riegel P, Provot C, Badel-Berchoux S, Jehl F, Olivares E, Hansmann Y. Kinetics of biofilm formation by Staphylococcus lugdunensis strains in bone and joint infections. Diagnostic microbiology and infectious disease. 2017 1;88(4):298-304.
CrossRef - Non LR, Santos CA. The occurrence of infective endocarditis with Staphylococcus lugdunensis bacteremia: a retrospective cohort study and systematic review. Journal of Infection. 2017 1;74(2):179-86.
CrossRef - Papapetropoulos N, Papapetropoulou M, Vantarakis A. Abscesses and wound infections due to Staphylococcus lugdunensis: report of 16 cases. Infection. 2013; 41:525-8.
CrossRef - H Obayes M, H Al-Charrakh A. Molecular study of Methicillin Resistant Staphylococcus lugdunensis (MRSL) Isolates in Hilla city, Iraq. karbala journal of pharmaceutical sciences. 2013 1;4(6):117-26.
- Mahmood N, Abdulrahman G. Prevalence of Staphylococci Among Dental Staff and Their Antibiotic Resistance Pattern. Al-Rafidain Dental Journal. 2023 20;23(1):140-9.
CrossRef - Muhammad H, Al-Mathkhury H. The Prevalence of methicillin resistant Staphylococcus aureus and methicillin resistant Staphylococcus epidermidis in AL-Sulaimania city. Iraqi Journal of Science. 2014;55(2A):386-93.
- Sulaiman AI, Abdulla BA. Detection of Biofilm Genes (IcaA and IcaD) in Staphylococcus Rafidain Journal of Scince. 2018 1;27,5/ pp.28-31,
CrossRef - Maki AA, Al-Taee AM. Bioremediation of Heavy Metals Using Staphylococcus in Shatt Al-Arab River. Iraqi Journal of Science. 2023 30:4971-81.
CrossRef - Mohammed, NI, Alwan MJ. Influence Immunization by Culture Filtrate Staphylococcus Aureus Antigen Carried by Silver Nanoparticles as Adjuvant Against Staphylococcus Aureus Infection in Mice. International Journal of Science and Nature, 8(3), 695-703.
- Mohammed, NI, Alwan MJ. isolation and identification of staphylococcus aureus strains from fresh and frozen meat in karbala province International Journal of Science and Nature, 2017b 8 (3), 704-709.
- Mitov I, Denchev V, Linde K. Humoral and cell-mediated immunity in mice after immunization with live oral vaccines of Salmonella typhimurium: auxotrophic mutants with two attenuating markers. Vaccine. 1992 1;10(1):61-6.
CrossRef - Jindal A, Sarkar S, Alam A. Nanomaterials-mediated immunomodulation for cancer therapeutics. Frontiers in chemistry. 2021 23; 9:629635.
CrossRef - Mohammed NI, Al-Awadi AQ. Immunopathological effect of whole sonicated Staphylococcus Lugdunensis Antigen uploaded with Zinc oxide nanoparticle in male albino Rats. African Journal of Biomedical Research. 2024 28;27(3):1230-47.
CrossRef - Taha L, Stegger M, Söderquist B. Staphylococcus lugdunensis: antimicrobial susceptibility and optimal treatment options. European Journal of Clinical Microbiology and Infectious Diseases. 2019 1; 38:1449-55.
CrossRef - Kaliniak S, Fiedoruk K, Spałek J, Piktel E, Durnaś B, Góźdź S, Bucki R, Okła S. Remodeling of Paranasal Sinuses Mucosa Functions in Response to Biofilm-Induced Inflammation. Journal of Inflammation Research. 2024 31:1295-323.
CrossRef - Hoyer, K. L., Wolff, D., Knop, C., Schell, U., Kola, A., Welte, T., and Schaffer, B. (2020). Invasive Staphylococcus lugdunensis infections: a retrospective analysis of 108 cases. European Journal of Clinical Microbiology & Infectious Diseases, 39(5), 943-951.
- Iwasaki Y, Fujio K, Okamura T, Yanai A, Yamamoto K. Toward therapeutic application of IL-10-producing regulatory T cells. Nihon Rinsho Men’eki Gakkai Kaishi= Japanese Journal of Clinical Immunology. 2013 1;36(1):40-6.
CrossRef - Jiang W, Li D, Zhang Y, Chang P, Zhu C, Shi Z, and Wu H. Audience response to an educational intervention about Staphylococcus lugdunensis Antimicrobial Resistance & Infection Control, 2019 8(1), 1-8.
- Jia G, Wang H, Yan L, Wang X, Pei R, Yan T, Zhao Y, Guo X. Cytotoxicity of carbon nanomaterials: single-wall nanotube, multi-wall nanotube, and fullerene. Environmental science & technology. 2005 1;39(5):1378-83.
CrossRef - ZhengY, Niyonsaba F, Ushio H, Nagaoka I, Fujimura T, and Ikeda S. Microbial invasion enhances the ability of mast cell-derived chymase to bind to and degrade extracellular matrix proteins: an effect associated with the pathogenesis of invasive peridontitis and arthritis. Cellular Immunology, 2008. 253(1-2), 214-224.
- Guzik K, Bzowska M, Smagur J, Krupa O, Sieprawska M, Travis J, Potempa J. A new insight into phagocytosis of apoptotic cells: proteolytic enzymes divert the recognition and clearance of polymorphonuclear leukocytes by macrophages. Cell Death & Differentiation. 2007;14(1):171-82.
CrossRef
- Gliga AR, Skoglund S, Odnevall Wallinder I, Fadeel B, Karlsson HL. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag release. Particle and fibre toxicology. 2014; 11:1-7.
CrossRef - Liu PY, Huang YF, Tang CW, Chen YY, Hsieh KS, Ger LP, Chen YS, Liu YC. Staphylococcus lugdunensis infective endocarditis: a literature review and analysis of risk factors. Journal of Microbiology, Immunology and Infection. 2010 1;43(6):478-84.
CrossRef - Gasch O, Camoez M, Dominguez MA, Padilla B, Pintado V, Almirante B, Molina J, Lopez-Medrano F, Ruiz E, Martinez JA, Bereciartua E. Predictive factors for mortality in patients with methicillin-resistant Staphylococcus aureus bloodstream infection: impact on outcome of host, microorganism and therapy. Clinical Microbiology and infection. 2013 1;19(11):1049-57.
CrossRef - Nesher L, Tarrand J, Chemaly RF, Rolston KV. Staphylococcus lugdunensis infections, filling in the gaps: a 3-year retrospective review from a comprehensive cancer center. Supportive Care in Cancer. 2017; 25:1063-9.
CrossRef - Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, Herridge M, Randolph AG, Calfee CS. Acute respiratory distress syndrome. Nature reviews Disease primers. 2019 14;5(1):18.
CrossRef - Reutershan J, Ley K. Bench-to-bedside review: acute respiratory distress syndrome–how neutrophils migrate into the lung. Critical care. 2004; 8:1-9.
CrossRef







